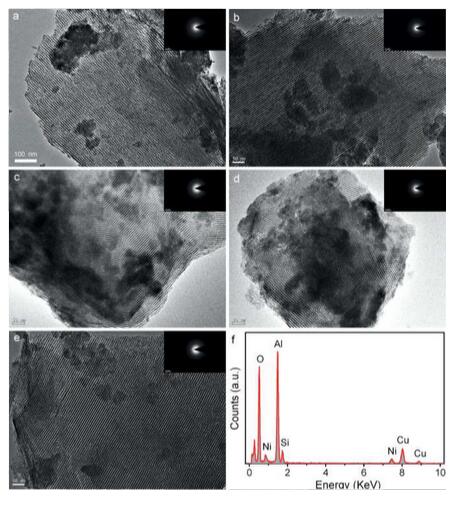
Abstract: Ordered mesoporous NiSiAl composite oxides with an amorphous phase were prepared by an improved evaporation-induced self-assembly strategy. The silica was distributed homogeneously in the mesoporous skeleton and improved the ordered mesoporous structure of NiAl. Catalytic tests toward the CO2 reforming of CH4 indicated that the NiSiAl exhibited higher catalytic activity and stability over 100 h at 700 degrees C than NiAl. The enhanced performance results from the high thermal stability induced by Si doping, which hinders the Al2O3 phase transformation from amorphous to gamma-Al2O3; therefore, the uniform dispersion of the Ni nanoparticles is retained owing to the confinement effect of the ordered mesopores. The stabilized Ni nanoparticles reinforced the resistance of the catalyst to carbon deposition and, thus, boosted its durability. Notably, even under more stringent conditions (750 degrees C, 200 h), NiSiAl-0.10 retained its amorphous Al2O3 structure and showed outstanding durability, almost without any deactivation. KeyWords Plus: SUPPORTED RHODIUM CATALYSTS; CARBON-DIOXIDE; SYNTHESIS GAS; METAL-OXIDES; NICKEL-CATALYSTS; COMPOSITE OXIDES; METHANE; NI; ALUMINA; DEACTIVATION Published in EUROPEAN JOURNAL OF INORGANIC CHEMISTRY, (21):3396-3404; 10.1002/ejic.201600463 JUL 2016
|