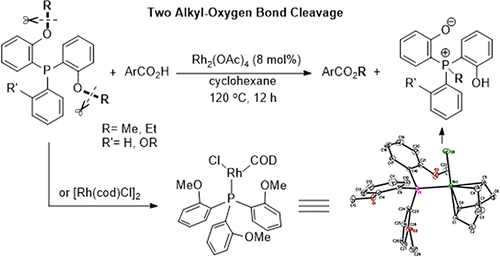
Abstract: An unprecedented rhodium-catalyzed selective cleavage of double alkyl-oxygen bonds of bis/tris(o-alkyloxyphenyl)phosphine has been realized, in which P atom functions as a. directing group and simple aryl acids are the methyl group acceptor to provide methyl esters and a quaternary phosphonium salt. The preliminary mechanism was investigated via an O-18 labeling experiment and stoichiometric reaction between a Rh-A crystal and an aromatic acid. KeyWords Plus:C-O BONDS; ACTIVATION/CROSS-COUPLING REACTION; OXIDATIVE ADDITION; METHYL ETHERS; CARBON-OXYGEN; GRIGNARD-REAGENTS; REDUCTIVE CLEAVAGE; TRANSITION-METALS; HYDRIDE COMPLEXES; DEMETHYLATION Published in ORGANOMETALLICS, 35 (19):3406-3412; 10.1021/acs.organomet.6b00638 OCT 10 2016
|