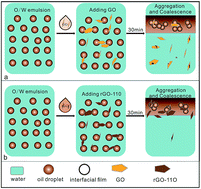
Abstract: A series of reduced graphene oxide (rGO) materials was synthesized by simple, clean, and controlled hydrothermal reduction of graphene oxide (GO). The chemical composition and properties of the obtained rGO materials were characterized by FT-IR, UV-visible absorption spectroscopy, XPS, AFM, zeta potential measurement, and interfacial tension analysis. As two-dimensional surfactants, various samples of rGO were employed to demulsify an oil-in-water emulsion. The demulsification performance of various demulsifiers was found to be considerably improved with increasing the reduction degree of the GO. In particular, the demulsification efficiency could reach about 99.97% for the rGO-110 sample. Quantum chemical calculations with a high quantum level of density functional theory with the empirical dispersion corrections approach (DFT-D) were performed to understand the mechanism of demulsification. The results revealed that rGO has a stronger adsorbability for asphaltene molecules than GO does by p-p interaction. Due to the strong pi-pi interaction between the rGO nanosheets and the asphaltenes, the protective film stabilizing the oil-in-water emulsion was more easily destroyed, thus promoting the oil droplets to coalesce to realize the separation of oil from water. KeyWords Plus: EXFOLIATED GRAPHITE OXIDE; CRUDE-OIL; MOLECULAR-DYNAMICS; SEPARATION; FILMS; ASPHALTENE; REDUCTION; RECOVERY; BREAKING; BITUMEN Published in RSC ADVANCES, 6 (108):106297-106307; 10.1039/c6ra18898b 2016
|