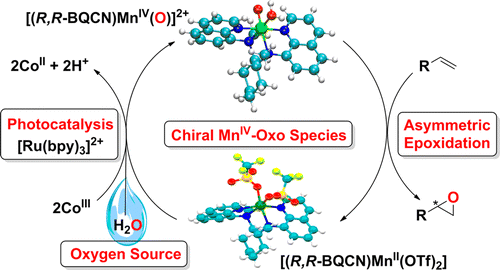
Abstract: Photocatalytic enantioselective epoxidation of terminal olefins using a mononuclear non-heme chiral manganese catalyst, [(R,R-BQCN)Mn-II](2+), and water as an oxygen source yields epoxides with relatively high enantioselectivities (e.g., up to 60% enantiomeric excess). A synthetic mononuclear non-heme chiral Mn(IV)-oxo complex, [(R,R-BQCN)Mn-IV(O)](2+), affords similar enantioselectivities in the epoxidation of terminal olefins under stoichiometric reaction conditions. Mechanistic details of each individual step of the photoinduced catalysis, including formation of the Mn(IV)-oxo intermediate, are discussed on the basis of combined results of laser flash photolysis and other spectroscopic methods. KeyWords Plus: ELECTRON-DEFICIENT OLEFINS; IRON-CATALYSTS; HYDROGEN-PEROXIDE; ENANTIOSELECTIVE EPOXIDATION; OXO COMPLEXES; METAL-OXO; C-H; OXIDATION; H2O2; REACTIVITY Published in JOURNAL OF THE AMERICAN CHEMICAL SOCIETY, 138 (49):15857-15860; 10.1021/jacs.6b10836 DEC 14 2016
|