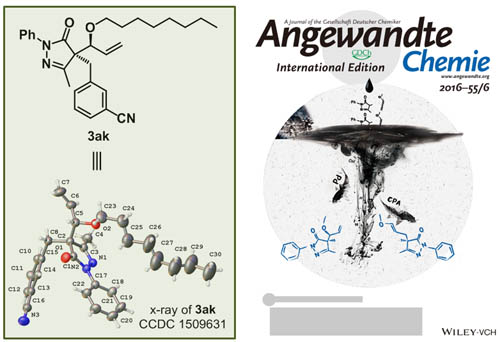
Abstract: A highly enantioselective regiodivergent addition of alkoxyallenes to pyrazolones was developed to afford multiply functionalized alkylated products bearing a quaternary carbon stereocenter in high yields with excellent stereoselectivities. One approach is enabled by palladium catalysis, thus leading to branched allylic pyrazol-5-ones under mild reaction conditions. The other is catalyzed by a chiral Bronsted acid to give linear products exclusively. Moreover, the usefulness of this new method was highlighted by converting the allylic products into other interesting multifunctionalized pyrazolone derivatives which would be of great potential for the exploitation of pharmaceutically important molecules. KeyWords Plus: CHIRAL PHOSPHORIC-ACID; WAGNER-MEERWEIN SHIFT; ASYMMETRIC-SYNTHESIS; ALLENES; CONSTRUCTION; DERIVATIVES; HYDROALKOXYLATION; PYRAZOL-5-ONES; STEREOCONTROL; COMPLEXES Published in ANGEWANDTE CHEMIE-INTERNATIONAL EDITION, 56 (4):1077-1081; 10.1002/anie.201610473 JAN 19 2017 |