Breakthrough has been achieved by the research group led by Prof. ZHANG ZhaoZhu of the Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, in the facile fabrication of super-hydrophobic copper surface through a process combining both electroless galvanic deposition and self-assemble of n-octadecanethiol. Superhydrophobicity with a static water contact angle of about 169 ± 2° and a sliding angle of 0 ± 2° was achieved.
As the reaction time prolong, the topography of the Cu surface changed from nanoparticle to dendritic nanostructure. In addition, the stability of the superhydrophobic surface was tested under the following tree conditions: 1) in different pH value from 1 to 13; 2) after freezing treatment at -20°C; 3) at ambient temperature. It shows a notable stability that the contact angle of the sample still remained higher than 150° in different conditions. It can be concluded that our approach can provide an alternative way to fabricate stable superhydrophobic materials.
The work has won support from the National Science Foundation of China (Grant No. 50835009 and Grant No.50721062).And it is published in the recent issue of Langmuir(Langmuir 2010, 26(5), 3654–3658).
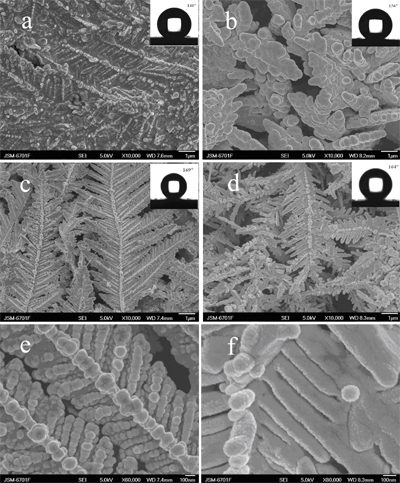
Effect of the deposition time on the morphology of the substrate (a) 10 secs, (b) 20secs, (c) 30 secs, (d) 60 secs, (e,f) larger view of c and d; contact angle shown in inset picture
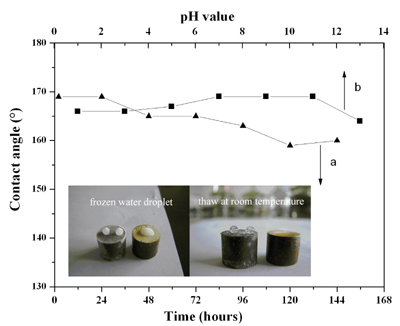
The variation of the apparent contact angle of the as-prepared superhydrophobic surface with different freezing time at -20°C (a) and Contact angles of water droplets with different pH values on the prepared superhydrophobic surface (b) inset picture shows the frozen water droplet on the surface


