Structural units containing multiple contiguous stereogenic centers are commonly found in various natural products and chiral drugs. Their construction represents a big challenge in organic synthesis. The highly selective construction of over three contiguous stereogenic centers in acyclic compounds is even more challenging. The development of a highly efficient asymmetric catalytic method is one of the most effective ways for the preparation of such kind of compounds.
Taking the advantage of the fact that imine can trap chiral enolate generated in situ in asymmetric Michael addition reaction, the research group headed by Prof. XIA Chungu and Prof. HUANG Hanmin of the Sate Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), CAS, has designed a kind of highly efficient asymmetric tandem reaction in cooperation with Prof. HU Xinquan of Zhejiang University of Technology.
With easily obtained α,β-unsaturated ketones, imines and alkyl zinc as starting materials and chiral copper complexes as catalysts, the construction of three contiguous acyclic stereogenic centers in keto-amine compounds can be achieved through tandem reaction in one step with high stereoselectivity. This protocol has been successfully applied in the highly selective synthesis of chiral isoindolinones and azetidines with multiple stereocenters. Chiral isoindolinones and isoindolinones derivatives are of great significance in biological science and medicinal chemistry. Azetidines possess various important biological properties such as activity against influenza virus.
The work was supported by the National Natural Science Foundation of China , and was published in the latest issue of Angew. Chem. Int. Ed. (Angew. Chem. Int. Ed. 2010, 49, 2728 http://www3.interscience.wiley.com/cgi-bin/fulltext/123316365/PDFSTART).
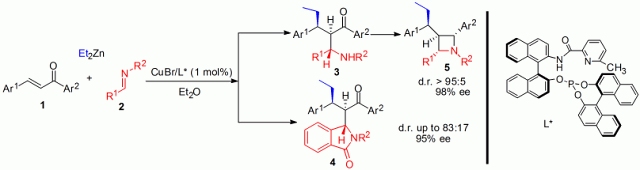
Copper-catalyzed tandem conjugate addition/Mannich reaction. L*=chiral ligand.


X-ray structures of the enantiomerically pure isoindolinone 4a (left) and azetidine 5a (right). The thermal ellipsoids are drawn at the 50% probability level. Blue N, green Br, red O, yellow S.


