Lewis acid-catalyzed C-H functionalization.(Picture by HUANG Hanmin et al)
Researchers of the State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), CAS, have successfully developed the Lewis acid-catalyzed C-H functionalization of 2-substituted azaarenes with N-sulfonylaldimines.
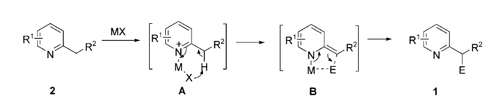
Based on their previous research on Pd-catalyzed benzylic addition of 2-methyl-azaarenes to aldimines (J. Am. Chem. Soc. 2010, 132, 3650-3651), researchers have successfully identified commonly used Lewis acids, such as AlCl3, FeCl3, BF3·Et2O and Sc(OTf)3, as versatile catalysts for the benzylic addition of 2-substituted azaarenes to N-sulfonylaldimines to afford heterocycle-containing amines via C-H bond activation. This protocol provides a reliable and rapid approach for the synthesis of isoindolinones and isoindolines that are of tremendous importance in medicinal chemistry via the C-H bond functionalization-triggered tandem reactions. This chemistry provides a mechanistically novel and synthetically useful transformation of azaarenes to important medicinal heterocycles.
Since the pyridine unit is an extremely important pharmacophore that can be found in many natural and synthetic bioactive compounds, numerous methodologies have been developed to access substituted pyridine derivatives. The research reports an efficient Lewis acid-catalyzed C-H functionalization of 2-substituted azaarenes with imines under neutral conditions. Notably, in a one-pot manipulation, nitrogen heterocycle-containing isoindolinones and isoindolines could also be obtained in high yields via C-H functionalization-triggered tandem reactions.
The research has received support from the National Natural Science Foundation of China and Chinese Academy of Sciences. The findings have been published in Adv. Synth. Catal. (Adv. Synth. Catal. 2010, 352, 3195 – 3200).
Further reading:
Progress in Research on Palladium-Catalyzed C-H Bond Activation


