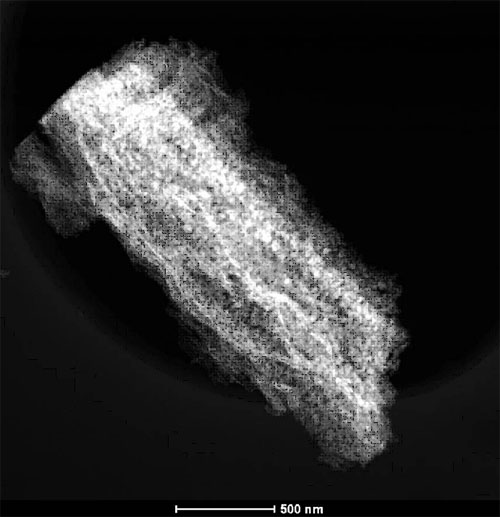
HAADF- STEM image of sample layered parallel Folding nanostructures prepared by calcination of layered parallel folding cobalt oxalate nanostructures precursor at 400℃for 3 h (Image by WANG Qihua et al.)
Researchers at the R&D Center of Lubricating and Protecting Materials of the Lanzhou Institute of Chemical Physics have selectively prepared one-dimensional and layered parallel folding of cobalt oxalate nanostructures by a one-step, template-free, water-controlled precipitation approach by simply altering the solvents used at ambient temperature and pressure.
Encouragingly, the feeding order of solutions played an extraordinary role in the synthesis of nanorods and nanowires. After calcination in air, the as-prepared cobalt oxalate nanostructures subsequently convert to porous Co3O4 nanostructures while maintaining their original frame structure. To shed light on their potential applications, the as-prepared Co3O4 nanostructures have been applied in electrode materials for supercapacitors, which exhibit large specific capacitances and good cyclability.
The easy synthesis and short production process would be helpful in production of Co3O4 nanostructures on a large scale. It not only could be extended to the synthesis of other metal oxides with controlled shape and structure but also provided a new route for the fabrication of inorganic materials into well-defined morphologies with potential applications.
As one of the solid precursors, cobalt oxalates are potential candidates that can be used as precursor salts to produce nanoporous Co3O4 due to their easy synthesis, low cost, good structure stability, and relatively low decomposition temperature in air. The micro emulsion or solvothermal methods have been developed to synthesize cobalt oxalate with controllable shapes. However, preparation of those nanostructures using these methods involves relatively complicated synthetic processes, such as a long time of solvothermal treatment, surfactants, and ion liquid. Besides, they cannot be produced on a large scale and cheaply, which largely hinders their practical use. Overall, it is more desirable to develop an alternative method to replace the micro emulsion or solvothermal methods for synthesizing cobalt oxalate with controllable shapes.
The work has received support from the National Foundation for Distinguished Young Scholars of China, Science Fund for Creative Research Groups of the National Natural Science Foundation of China and National Program on Key Basic Research Project of China. The findings have been published in Inorg. Chem. 2011, 50, 6482–6492 (Inorg. Chem., 2011, 21, 9073–9078).


