Supported Copper(II)-Catalyzed Azide-Alkyne Cycloaddition in Water (Image by XIA Chungu, LIU Jianhua et al.)
The copper-catalyzed azide-alkyne cycloaddition reaction is a representative example of click chemistry, which proceeds with high regioselectivity when terminal alkynes are used and provides 1,2,3-tri-azoles with 100% atom efficiency. This important reaction has attracted a considerable amount of attention in organic synthesis, medicinal chemistry, surface and polymer chemistry, as well as bioconjugation applications.
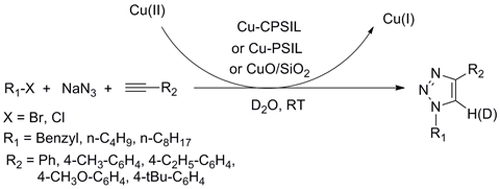
Researchers at the State Key Laboratory for Oxo Synthesis and Selective Oxidation of the Lanzhou Institute of Chemical Physics (LICP) have successfully prepared three immobilized Cu(II) catalysts, namely cross-linked polymeric ionic liquid material-supported copper (Cu-CPSIL), imidazolium-loaded Merrifield resin-supported copper (Cu-PSIL) and silica dispersed CuO (CuO/SiO2). These catalysts have been proved to be efficient catalysts for the one-pot synthesis of 1,4-disubsituted-1,2,3-triazoles by the reaction of alkyl halides with sodium azide and terminal alkynes in water at room temperature. Moreover, these supported copper catalysts were recovered quantitatively from the reaction mixture by simple filtration and reused for five consecutive recycles without significant loss of catalytic activity. In addition, water was used as the reaction media and the proton provider, the latter was found to be very important for the reaction. Based on XPS, IR and ESI-MS characterizations, a mechanism was proposed as follows: the alkyne homocoupling proceed on the Cu(II) site to form the catalytically active Cu(I) species, and the cycloaddition proceeded over the active Cu(I) species generated.
It is expected that the excellent nature of these immobilized, supported copper catalysts will be helpful in understanding heterogeneous Cu(II) catalysts in click reactions.
The work has received support from the National Natural Science Foundation of China (grant no.20625308, 21073209). The findings have been published in Adv. Synth. Catal. (Adv. Synth. Catal. 2011, 353, 1534-1542).


