Carbon material has been a hot topic in the field of material research due to its excellent performance. Scientists both home and abroad have done a lot of research on the controllable preparation of novel nano-carbon materials and their applications in supercapacitors and other chemical energy storage devices.
The research group headed by Professor YAN Xingbin at the State Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences (CAS), has been engaged in developing novel environmental friendly nano-carbon materials with wide operating temperature, high energy density and power density since 2009.
Their recent investigation on the influence of molar concentration of ionic liquid electrolyte and temperature on the supercapacitive performance of graphene sheet reveals that GNSs exhibit better capacitive behavior in 2 M ionic liquid electrolyte. It is also found that, the GNSs electrode shows better supercapacitive performance when the operating temperature ranges from -20 oC to 60 oC in the EMIMBF4 electrolyte, and its specific capacitance shows weak temperature dependence. The results have been published in J. Mater. Chem.(J. Mater. Chem.2012, 22, 8853).
Previously, the research group has studied the influence of organic solvents and the alkyl chain length of alkylimidazolium tetra?uoroborate ionic liquid electrolytes on the electrochemical behavior of GNSs. The results reveal that from series of organic solvents, in 1-ethyl-3-methylimidazolium tetrafluoroborate (EMIMBF4)/N,N-dimethylformamide (DMF, C3H7NO) electrolyte the GNS electrode shows the best electrochemical performance. The work has been published in J. Mater Chem.(J. Mater Chem.2011, 21, 13205), and highlighted by the journal. In addition, the research group has prepared the graphene film and the shape memory electrode material via the self-assembly and composite technology, respectively. These materials show excellent capacitance performance. The findings have been published in Electrochim. Acta.(Electrochim. Acta.2012, 60, 41) and J. Power. Sources.(J. Power. Sources.doi: 10.1016/j.jpowsour.2012 03. 086) respectively.
The work has received support from the National Natural Science Foundation .
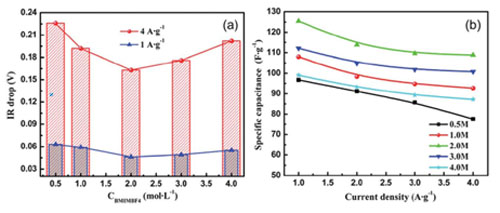
Fig.1 (a) The ‘‘IR drop’’ of the GNSs electrode as a function of the molar concentration of BMIMBF4 electrolyte, (b) The speci?c capacitance of the GNSs electrode in the EMIMBF4 electrolyte with different molar concentrations as a function of the current density. (Image by YAN Xingbing et al.)
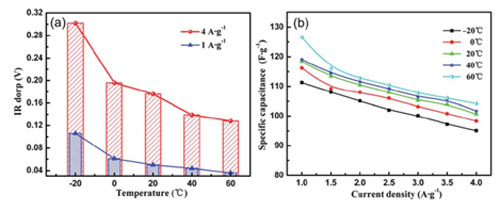
Fig.2 (a) The ‘‘IR drop’’ of the GNSs electrode as a function of different temperature in 2 M BMIMBF4 electrolyte, (b) The speci?c capacitance of the GNSs electrode in 2 M EMIMBF4 electrolyte at different temperature as a function of the current density.(Image by YAN Xingbing et al.)


