As a new kind of energy storage devices, supercapacitors possess a higher power density, a longer cycle life, a simpler charging circuit, no memory effect and are much safer and maintenance-free. They have broad applications in electric vehicles, mobile communications, defence-related science and technology, and so on.
The tribology of low dimensional materials research group at the Lanzhou Institute of Chemical Physics of the Chinese Academy of Sciences has made new findings in graphene nanosheets/Ionic liquid based supercapacitors.
Researchers have thoroughly investigated the electrochemical properties of graphene nanosheets (GNSs) in alkylimidazolium tetrafluoroborate ionic liquids/organic solvent electrolytes using electrochemical measuring techniques, especially the influence of organic solvents with different functional groups on the electrochemical properties of the GNSs. Based on the experimental results, researchers have selected ionic liquid/organic electrolytes with excellent properties. In addition, they have evaluated the effect of the alkyl chains on the imidazole ring in ionic liquids on the electrochemical properties of GNSs, constructed a structural model of ionic liquid/GNS electrode interface and studied the cycling stability of GNS in ionic liquid/organic solvent electrolyte.
The electrochemical measuring results show that as electrodes GNS exhibits a wide potential window of 1.9 V in ionic liquid/organic solvent electrolytes, much higher than that in aqueous electrolytes. It also possesses higher energy density. With the increasing of the alkyl chain length, the electrochemical property of GNS in ionic liquid/organic solvent electrolyte becomes worse. After 1500 cycles, the capacity retention of GNS in ionic liquid/organic solvent electrolyte maintains original capacity, namely 120.8% and shows excellent cycling stability as well.
The work is significant for revealing the structure and property of ionic liquid/electrode interface and provides a new choice for improving the potential window and energy density of supercapacitors.
The above work has been published in J. Mater. Chem. (2011, 21, 13205–13212). The publication has become one of the hot articles of the journal has been highlighted on the online version of the journal.
The work has received support from the National Nature Science Foundation of China and the Postdoctoral Science Foundation of China.
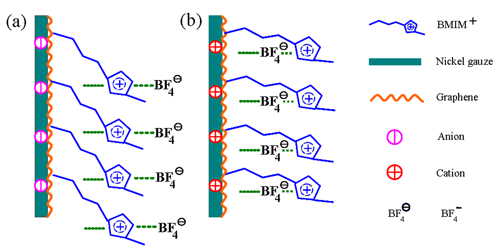
A schematic showing the collocation of anions and cations at the GNS electrode/ionic liquid interface during the charging (a) and the discharging (b) processes. (Image by YAN Xingbin et al.)
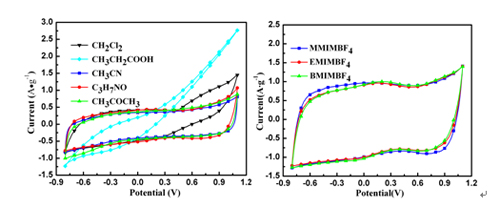
CV curves of the GNS electrode in EMIMBF4/organic electrolytes (left) and CV curves of the GNS electrode in ILs/DMF (right). (Image by YAN Xingbin et al.)


