Researchers at the State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences (CAS), have developed novel methods for transition-metal-catalyzed C-H activation/addition reactions.
Transition-metal-catalyzed C-H additions to unsaturated C=X (X=C, O, N) bonds by C-H bond activation has become an important strategy for the construction of C-C bonds. Based on their first research on Pd-catalyzed benzylic addition of 2-methyl-azaarenes to aldimines (J. Am. Chem. Soc.2010, 132, 3650-3651), they have successfully developed several protocols for this type of sp3 C-H activation/addition reactions (Adv. Synth. Catal.2010, 352, 3195–3200.; Org. Lett.2011, 13, 2580–2583). Recently, they have developed a rhodium-catalyzed selective sp2 C-H activation and subsequent conjugate addition to α,β-unsaturated ketones under mild reaction conditions. The findings have been published in Chem. Eur. J. (Chem. Eur. J.2012, 18, 9511 – 9515).
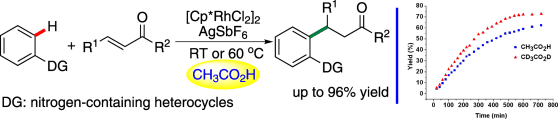
Rhodium-catalyzed selective C-H activation/addition to α,β-unsaturated ketones (Image by HUANG Hanmin et al.)
Vital to the success of this approach is the rational analysis of the proton-transfer effect involved in this conjugate addition reaction. With CH3CO2H as the solvent, the inherent substrate inhibition effect of this type of reaction could be successfully overcome allowing the reaction proceed smoothly under mild reaction conditions. This approach provides a new strategy to accomplish this type of C-H activation/addition process.
Besides, researchers developed a Lewis acid-catalyzed conjugate addition of 2-alkylazaarenes to methylenemalononitriles through sp3 C-H bond functionalization, which provides an efficient and reliable method for incorporation of the nitrile group into the heterocycles. This protocol provides an efficient synthetically useful approach for transformation of azaarenes to valuable medicinal heterocycles. The work has been published in Adv. Synth. Catal. ( Adv. Synth. Catal.2012, 354, 2146 – 2150).
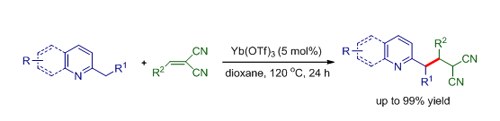
Yb(OTf)3 catalyzed addition of 2-substituted azaarenes to methylenemalononitriles (Image by HUANG Hanmin et al.)
The work was supported by the National Natural Science Foundation of China and CAS.


