Hydrogels are three-dimensional (3D) structured networks which can be prepared from virtually any water-soluble polymer, encompassing a wide range of chemical compositions and bulk physical properties.The unique properties of hydrogels have sparked particular interest in their numerous potential applications, ranging from traditional swelling–deswelling, tunable responsiveness and drug delivery, to increasing attention for remote controlled actuators, cell encapsulation and tissue engineering.However, hydrogel prepared through conventional aqueous solution polymerization is colloid and need to be granulated which requires high energy consumption for drying.
The functional composite materials research group at R&D Center for Eco-material and Eco-chemistry, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences (CAS), has prepared chitosan grafted poly(acrylic acid)/unexpanded vermiculite (CTS-g-PAA/UVMT), a granular composite hydrogel with three-dimensional networks via in situ graft polymerization.
By making use of the chemical activity of natural polymer chitosan and the fact that it shows positive charge under certain circumstance, researchers have prepared the granular composite hydrogel under an ambient temperature in air atmosphere.The findings have been published in Chemical Engineering Journal(2012, 179(1): 90-98) and Chemical Engineering Journal(2012, 200-202: 601-610).
In addition, they have prepared a granular hydrogel based on chitosan (CTS) and acrylic acid (AA) using Fenton’s reagent as the redox initiator and N,N'-methylenebisacrylamide as the crosslinker under ambient conditions.
The formed 3D network can serve as a micro- or nano-reactor for the production of highly stable silver nanoparticles by the in situ reduction of Ag+ ions with NaBH4 as reducing agent and Al3+ as surface crosslinking agent. The swollen 3D polymeric matrix can accommodate more Ag nanoparticles, while the higher surface crosslinking can stabilize these particles against escape. The findings have been published in J. Mater. Chem.(2012, 22, 16552-16559).
The work has received support from the National Natural Science Foundation of China.
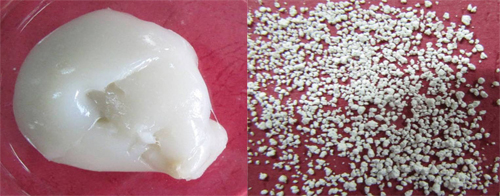
Colloidal hydrogel and granular hydrogel (Image by WANG Aiqin et al.)
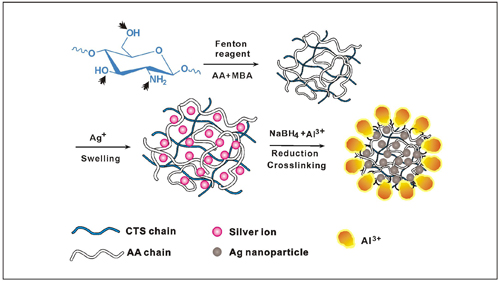
Schematic representation for the Ag nanoparticles formation within the hydrogel network. (Image by WANG Aiqin et al.)


