1,3-diamines are important structural motifs in many natural products and pharmaceuticals. As a result, much effort has been devoted to the development of effective methods to access these compounds. However, unlike their 1,2-diamine counterparts, few methods for the direct synthesis of 1,3-diamines have been described.
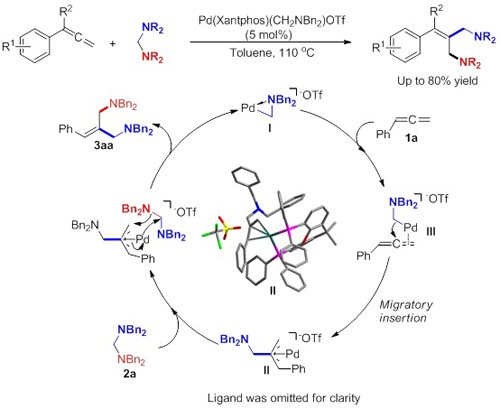
Palladium-catalyzed difunctionalization of allenes. (Image by HUANG Hanmin et al.)
The research group headed by Professor HUANG Hanmin from the State Key Laboratory for Oxo Synthesis and Selective Oxidation at the Lanzhou Institute of Chemical Physics (LICP) of the Chinese Academy of Sciences (CAS) has developed a new and effective method to synthesize 1,3-diamines.
They have developed the first direct insertion of an allene into an aminal C-N bond to provide 1,3-diamines through a palladium-catalyzed C-N bond activation. A unique cyclometalated PdII complex has been identified as an efficient catalyst for this reaction.
This new transformation may be used for the coupling of a broad range of substrates and thus represents a concise, operationally simple, and useful method for the preparation of 1,3-diamines that are of interest in synthetic organic chemistry. Mechanistic studies suggest that the reaction proceeds via a p-allylpalladium complex, which has promise as a valuable intermediate in many other reactions.
The work has been published in Angew. Chem. Int. Ed.(Angew. Chem. Int. Ed.2014, DOI: 10.1002/anie.201403774).


