Formaldehyde is a major organic pollutant and can cause great damage to human health. Current formaldehyde elimination methods focus on absorption and catalytic oxidation of indoor gaseous formaldehyde with low density. These methods cannot meet the needs of oxidative elimination of high-density formaldehyde in industrial waste water. Moreover, with these methods, formaldehyde is converted into CO2, a major component of greenhouse gases, and water. Therefore, to develop a highly efficient technique for the selective catalytic oxidation of formaldehyde is an urgent problem to be solved.
Schematic illustration of the catalytic oxidation of formaldehyde for hydrogen production over metallic Pd nanotubes.(Image by BI Yingpu et al.)
The work has received support from the National Natural Science Foundation of China.
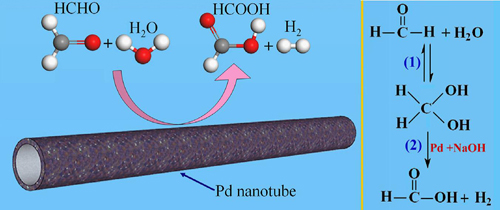
The research group headed by Professor BI Yingpu at the Lanzhou Institute of Chemical Physics (LICP) of the Chinese academy of Sciences (CAS) has developed a facile and efficient strategy for facilitating the hydrogen production from HCHO aqueous solution by using hollow Pd nanotube as the catalysts at room temperature. The hollow Pd nanotubes could serve as a highly efficient and convenient catalyst for inhibiting Cannizzaro reaction and facilitating hydrogen generation from alkaline formaldehyde solution at room temperature. This hollow nanostructure exhibits much higher capability and stability for hydrogen production than conventional Pd nanoparticles. By further optimizing the reaction parameters, such as reaction temperature, formaldehyde concentrations, and NaOH concentrations, the hydrogen generation rates could be further increased to as high as 170ml.min-1.
In the present hydrogen production system, H2 was the only gaseous product in all catalytic experiments and at all temperature, and other gases such as CO or CO2 were not generated. Because of the low costs of reagents and the mild reaction conditions, besides formaldehyde, with the strategy, other aldehydes, including acetaldehyde, propanal, and benaldehyde, could also produce hydrogen with high efficiencies.
The method is of guiding significance for the development of efficient oxidative elimination techniques of organic aldehyde pollutants.
The findings have been published in Nano Energy(Nano Energy, 2014, 8, 103).


