Nanosizing is the fashionable method to obtain a desirable electrode material for energy storage applications. Thus a question arises: do smaller electrode materials exhibit better electrochemical performance?
The research group headed by Professor YAN Xingbin at the Lanzhou Institute of Chemical Physics (LICP) of the Chinese Academy of Sciences has provided different results in their recent study.
Nanomaterials used in electrochemical energy storage devices have been synthesized by various methods. However, a precisely size-controlled synthesis and a systematic study of size-dependent electrochemical performance for the electrode materials are rarely performed due to the difficulty in controlling the size, particularly when the size is less than 10 nm.
In YAN’s study, they have, for the first time, prepared a series of ultra-small Ni(OH)2 nanoparticles with tunable sizes ranging from 3.3 to 12.2 nm via a scalable formation and disassociation of a nickel-citrate complex. These size-controlled Ni(OH)2 nanoparticles overcome the previous size limitation and could be a desirable platform to reveal the nanosize effect on the electrochemical performance of sub-10-nm electrode materials. Then, they carried out electrochemical measurements of the prepared size-controlled Ni(OH)2 nanoparticles.
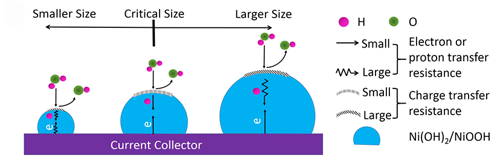
Model depicting the relationship between the impact factors and particle size in the discharging process of Ni(OH)2 nanoparticles. (Image by YAN Xingbin et al.)
The results show that the best electrochemical performance was observed with a specific capacity of 406 Cg-1, an excellent rate capability was achieved at a critical size of 7.9 nm and a rapid decrease in the specific capacity was observed when the particle size was less than 7.9 nm. Clearly, the nanosize effect on Ni(OH)2 nanoparticles presented here is different from the popular concept, that is, below the critical size, smaller Ni(OH)2 nanoparticles have a lower capacity due to the quantum confinement effect from the decreased conductivity and sluggish charge and proton transfer.
Thus, this work will help in understanding the nanosize effect on electrochemical behaviors and provides valuable information for selecting appropriately sized electrode materials to design high performance energy storage devices.
The work has been published in NPG Asia Materials(2015, 7, e183. doi:10.1038/am.2015.42).
Key words:electrode material; nanosize effect;electrochemical perform
Contact
YAN Xingbin
Laboratory of Clean Energy Chemistry and Materials, State Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou,
E-mail: xbyan@licp.cas.cn


