Land observing satellites and meteorological satellites fly in low earth orbit in which the most abundant species is atomic oxygen. move through atomic oxygen at high velocities, resulting in high fluxes of atoms being swept onto the forward facing surface with an effective translational energy of 5eV. This energy value is high enough to break chemical bonds of most materials commonly used in space applications (such as polymer and solid lubricants).
The researchers at the Lanzhou Institute of Chemical Physics (LICP) of the Chinese Academy of Sciences (CAS) have revealed the erosion mechanism of MoS2?based films, when they are exposed to atomic oxygen environments in cooperation with researchers from the Institute of Physics of the CAS.
They have exposed MoS2, titanium-doped and MoS2/Ti multilayer films to the atomic oxygen, and investigated the erosion mechanism of these films as a function of incident fluence by Rutherford back scattering (RBS) and focused ion beam combining with scanning electron microscopy (FIB&SEM).
The results reveal that the main erosion process of MoS2 film exposed to the atomic oxygen source is the formation and releasing of volatile species by the reaction of oxygen with sulfur atoms. The sulfur atoms are eroded by the incident atomic oxygen atoms and the removed sulfur amount increases but the erosion rate decreases with the increasing of incident fluence. For pure MoS2 films the erosion process turns to saturate at the end of investigated fluence of 4.8 × 1021 Ocm?2. For Ti-doped and MoS2/Ti multilayer films the saturation of sulfur erosion happens when the incident fluence reaches 5.2 × 1019 and 2.6 × 1019 Ocm?2 respectively, which is much earlier than that of pure MoS2 films. FIB cross-section results reveal that pores structures in the pure MoS2 films provide a reaction highway, which allows the incident atomic oxygen to be able to reach and react with the sulfur at bottom.
Introducing titanium doping or MoS2/Ti multilayer structures can reduce the density of pores and defects in the initial films. Consequently, the erosion process is suppressed or blocked, and the instinct lubricant properties of MoS2 phases can be well-retained in vacuum sliding conditions.
The work has received financial support from the National Natural Science Foundation of China and National Program on Key Basic Research Project (973 Program) funded by the ministry of Science and Technology of China.
The findings have been published in ACS Appl. Mater. Interfaces2015, 7, 12943?12950.
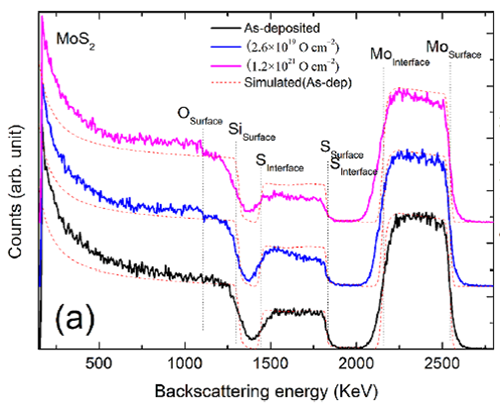
RBS spectra of (a) MoS2 films before and after atomic oxygen exposure to fluence of 2.6 × 1019 and 1.2 × 1021 O cm?2, respectively. (Image by LICP)
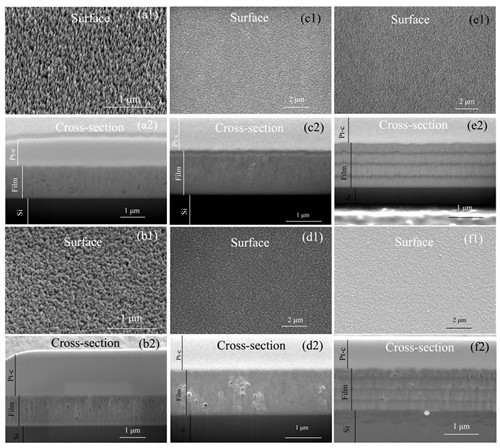
Surface and cross-section SEM images of as-deposited (a1, a2) MoS2, (c1, c2) Ti-doped MoS2, and (e1, e2) Ti/MoS2 multilayer films, after atomic oxygen exposed (b1, b2) MoS2, (d1, d2) Ti-doped MoS2, and (f1, f2) Ti/MoS2 multilayer films to a fluence of 2.1 × 1020 O cm?2.(Image by LICP)
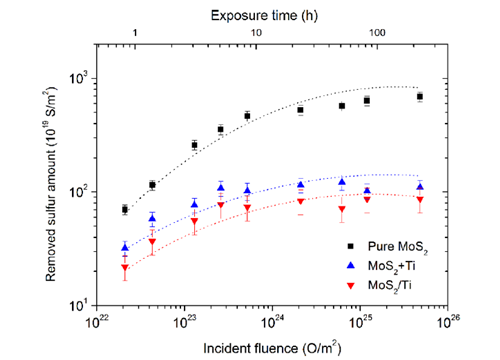
Removed sulfur amount of MoS2, Ti-doped MoS2, and MoS2/Ti multilayer films exposed to the atomic oxygen as a function of the incident fluence.(Image by LICP)
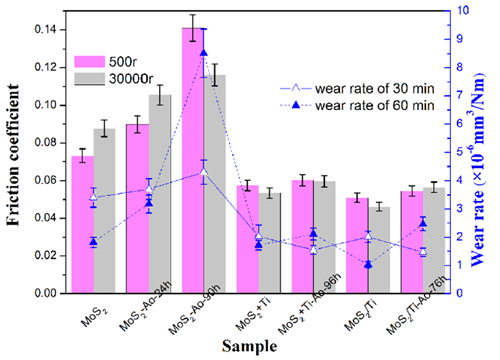
Mean friction coefficient of MoS2, Ti-doped, and MoS2/Ti multilayer films before and after atomic oxygen exposure for different exposure durations within 500r and 30000r tribo-tests.(Image by LICP)
Contact:
WANG Peng
State Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, China
E-mail: pengwang@licp.cas.cn


