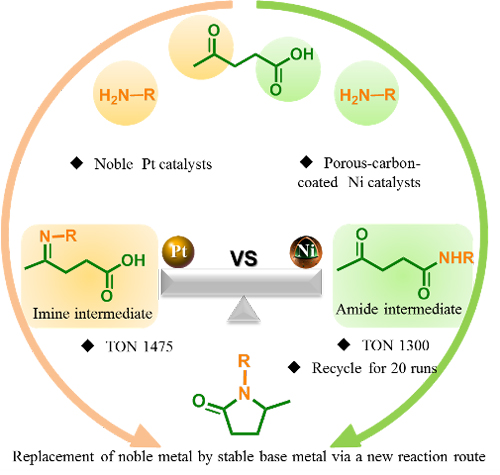
Replacement of noble metal by stable base metal via a new reaction route (Image by LICP)
In industry production, platform chemicals refer to those basic organic compounds with rich sources, low prices, multiple applications, and large yearly output (mostly more than 100,000 tons per year). A series of high value-added products, such as methane, ethanol, ethylene, lactic acid, and so on, can be synthesized from platform chemicals. The U.S. Department of Energy has selected 12 most representative platform chemicals, among which levulinic acid and furfural are important ones, from over 300 compounds derived from biomass.
Dr. Li Fuwei’s research group of the Lanzhou Institute of Chemical Physics (LICP) of the Chinese Academy of Sciences has been working on the catalytic conversion of levulinic acid and furfural in recent years. They have developed a series of non-noble metal based catalysts and turned the two chemicals into high value-added chemicals and fuels.
Levulinic acid has two functional groups (carbonyl and carboxyl) that can react with amine compounds, through which imine and amide are formed respectively. Li’s research group analyzed the activity of two kinds of catalysts, the noble metal Platinum and the non-noble metal Nickel in the hydrogenation of imines, and thus found an unconventional pathway via which Ni catalyzes the reductive amination of levulinic acid to form pyrrolidone. In this pathway, the amide intermediate is firstly produced, followed by cyclization, intramolecular dehydration, and hydrogenation, after which forms the pyrrolidone.
In addition, they have also cooperated with Qin Yong’s research group of the Institute of Coal Chemistry, Chinese Academy of Sciences, to improve the catalyst. They utilized the technology of Atomic Layer Deposition (ALD) and developed the highly stable porous-carbon-coated Ni catalysts. The coat is capable of efficiently preventing the loss and agglomeration of Ni NPs and generates no negative effect on the reaction’s activity. In the experiments, no loss or agglomeration of metal nanoparticles in the catalyst was found during 20 cycles of the reaction.
The results mentioned above show the possibility to produce pyrrolidone with levulinic acid using the non-noble Ni as the catalyst, a quite economic method, which can be applied in a wide range of industries. For other researchers, the progress made also provides a new way to develop non-noble metal catalysts by regulating the reaction pathway and to improve the stability of the heterogeneous catalyst in the liquid phase reaction. The research paper is published in ACS Catalysis, 2017, 7, 4927-4935.
LI Fuwei
State Key Laboratory for Oxo Synthesis & Selective Oxidation, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou,
E-mail: fuweili@licp.cas.cn


