Alkylboronic acids and their derivatives have found widespread applications in pharmaceuticals and synthetic chemistry. Accordingly, numerous methods have been developed to prepare these structures. Transition-metal-catalyzed hydroboration of alkenes is one of the most convenient and straightforward methods to synthesize these compounds with chemo- and regioselectivity. Because of the low reactivity of aliphatic internal alkenes and the difficulty of controlling the regioselectivity of hydroboration, only a few examples have been developed for regioselective hydroboration of aliphatic internal alkenes. In particular, the addition of a boryl group to the distal carbon remains a distinct challenge for aliphatic internal alkenes.
Recently, researchers from the Lanzhou Institute of Chemical Physics of the Chinese Academy of Sciences (LICP) report a highly distal selective hydroboration of aliphatic internal alkenes using readily available iridium dimer precursor [Cp*IrCl2]2 (Cp* = pentamethylcyclopentadienyl) as the catalyst in collaboration with Zhengzhou University and Nankai University. This unusual selectivity complements reported copper-, rhodium-, and iridium-catalyzed hydroboration. The current method could tolerate a variety of β,γ- and γ,d-unsaturated carbonyl compounds, affording the borylated products in good to excellent regioselectivity (up to 99:1). DFT calculations reveal the steric effect of Cp* and Bpin gives rise to the observed regioselectivity. In addition, a rare Ir(III/V) mechanism is proposed through calculations that is distinct from the generally accepted Ir(I/III) mechanism in hydroboration of alkenes.
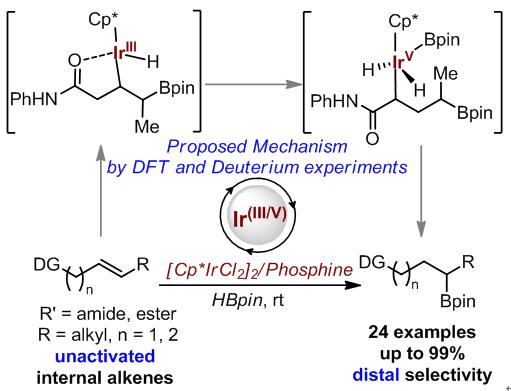
These findings are described in the article entitled ‘Iridium-Catalyzed Distal Hydroboration of Aliphatic Internal Alkenes’, recently published in the Journal Angewante Chemie International Edition (https://onlinelibrary.wiley.com/doi/pdf/10.1002/anie.201902464). This work was financially supported by the National Natural Science Foundation of China, Natural Science Foundation of Jiangsu Province, Lanzhou Institute of Chemical Physics, State Key Laboratory for Oxo Synthesis and Selective Oxidation.


