Since energy storage devices are often used in a magnetic field environment, scientists have explored how an external magnetic field affects the charge storage of nonmagnetic aqueous carbon-based supercapacitor systems.
Recently, an experiment designed by Prof. YAN Xingbin’s group from the Lanzhou Institute of Chemical Physics (LICP) of the Chinese Academy of Sciences has revealed that applying an external magnetic field can induce capacitance change in aqueous acidic and alkaline electrolytes, but not in neutral electrolytes. The experiment also shows that the force field can explain the origin of the magnetic field effect.
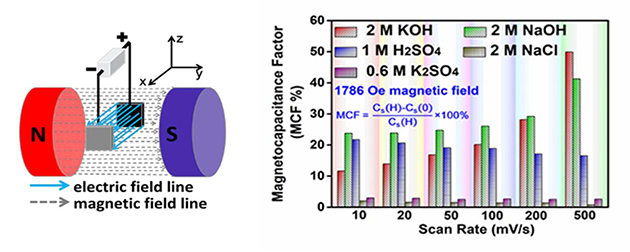
Figure 1. The drawing of test apparatus and capacitance changes at different scan rates in different electrolytes under magnetic field (Image by LICP)
This new discovery establishes a relationship between magnetic fields and supercapacitors, and provides insight into the transport behavior of ions in aqueous electrolytes.
Carbon-based supercapacitors are among the most prominent electrochemical energy storage devices because of their excellent power output and superior cycling life. During the charging/discharging process, the difference in electrical potential between the positive and negative electrodes generates a magnetic field based on Faraday’s law of electromagnetic induction.
Moreover, supercapacitors are often used in electronic equipment that generates a magnetic field as well. However, whether the magnetic field affects the charge storage of supercapacitors was still unknown.
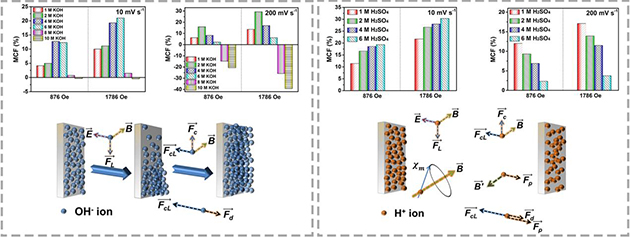
Figure 2. The variation of capacitances and the forced convection mechanism of ions in KOH and H2SO4 electrolytes under magnetic field (Image by LICP)
In this work, the researchers first reported that the external magnetic field indeed affects the charge storage of a nonmagnetic aqueous carbon-based supercapacitor system, thus breaks the negligible effect of the magnetic field on nonmagnetic electrochemical systems.
According to the researchers, the direction and intensity of the magnetic field, concentration of electrolytes and voltammetry sweep all affect the capacitance change in acidic and alkaline electrolytes.
In addition, a quantitative relationship among the limiting current density at the electrode/electrolyte interface, the intensity of the magnetic field, and the concentration and viscosity of the electrolytes was built, which provided a completely new insight into the charge transport behavior of supercapacitors.
“By establishing the relationship between magnetic fields and supercapacitors, we were able to deeply understand the transport behavior of ions in aqueous electrolytes. We expect to apply magnetic field-enhanced electrochemistry to other energy storage devices,” said Prof. YAN.
The results were published online in Cell Reports Physical Science in an article entitled “Magnetic field induced capacitance change of aqueous carbon-based supercapacitors.”
This work was funded by the National Natural Science Foundation of China, the Chinese Academy of Sciences, the Postdoctoral Science Foundation of China and the Zhaoqing Municipal Science and Technology Bureau of China.
Contact:
YAN Xingbin
Email: xbyan@licp.cas.cn
Lanzhou Institute of Chemical Physics


