As the interfacial water layer hinders the formation of interfacial contacts and intermolecular interactions between the adhesive and the substrate, in almost all cases of underwater adhesion, water molecules typically act as a destroyer, resulting in poor adhesion,. Thus, removing interfacial water from the substrate surfaces is essential for the formation of super-strong underwater adhesion. Despite various single physical attempts, such as absorption, hydrophobic repulsion and extrusion, this long-standing obstacle to the complete removal of interfacial water contradiction is hard to solve, rooted in their unavoidable presence of bound water in at the interface. Hence, an unconventional dehydration mechanism with a conceptually new material design at the physical-chemical coupling and a multiscale method are required to deep removal of water at the contact interface and self-adaptive cohesion enhancement to create powerful underwater adhesives.
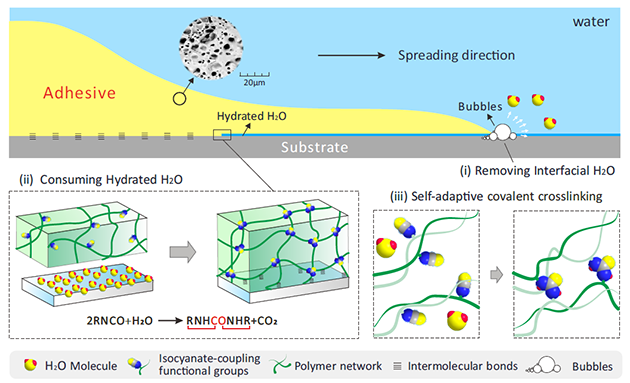
Figure 1. Design and adhesion process of the SLU-adhesive.
Recently, a study published in Proceedings of the National Academy of Sciences of the United States of America (PNAS), conducted by ZHOU Feng group from the Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences. A Self-strengthening Liquid Underwater Adhesive (SLU-adhesive) was developed, based on a physical-chemical coupling multiscale dehydration mechanism, achieving robust underwater adhesion by deep removal of water at the contact interface and self-adaptive cohesion enhancement.
In this work, the adhesion mechanism of the removal of interfacial water involves multiscale dehydration in three steps, namely physical replacement of the surface water on the substrate with SLU-adhesive at mm scale because of its excellent wettability, physical removal of water at μm scale by generating carbon dioxide bubbles from the chemical reaction between isocyanate groups and water at the interface, and simultaneous chemical consumption of the surface-bound water at molecular level.
This series of adhesion behaviors is instantaneous, spontaneous, and tacitly coordinated throughout the entire process of liquid adhesive contact, spreading, wetting, and gelation on the substrate surface, generating high underwater adhesion performance over 1,600 kPa. Strong adhesion can be achieved on various materials, including inorganic metals and organic plastic materials, without preloading in different environments such as pure water, a wide range of pH solutions (pH = 3 to 11), and seawater.
Due to the excellent adhesion property and self-adaptive adhesion procedure, SLU-adhesive materials demonstrate great potential for a wide range of applications in underwater sand stabilization, underwater repair, and even as a self-reporting adhesive for the detection of adhesion failure.
Contact:
Zhou feng
Email: zhouf@licp.cas.cn
Lanzhou Institute of Chemical Physics


