Biological enzymes exhibit high catalytic activity and selectivity in numerous catalytic reactions, such as non-heme enzymes that carrying out metabolic reactions in organisms. Among the reported biomimetic model compounds, tetradentate aminopyridine (N4) ligands have emerged as appealing frameworks because of their easy synthesis and facile diversification, and their complexes with metals such as Fe and Mn have proven to be versatile and powerful catalysts for a variety of (enantioselective) oxidation reactions.
The SUN Wei’s group from the Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, has been focusing on bioinspired manganese and iron complexes for enantioselective oxidation reactions for years. They have developed the chiral amino benzimidazole N4 manganese and iron complexes catalytic system which has shown remarkable success in asymmetric oxidation of C=C and C-H bonds(Acc. Chem. Res. 2019, 52, 2370-2381; Chem. Commun. 2020, 56, 13101- 13104; ACS Catal. 2021, 11, 10964-10973; J. Catal. 2022, 406, 87-95)
Recently, the research group has developed a series of Zn-N4 complexes from easily prepared aminopyridine N4 ligands that have been widely explored in biomimetic oxidations. These zinc complexes demonstrated excellent catalytic performance toward CO2 fixation with terminal epoxides under cocatalyst- and solvent-free conditions (Figure 1). Spectroscopic characterizations and control experiments revealed that catalyst Zn-3 with a DAP ligand appears to be a multifunctional catalyst due to releasing one pyridine for the activation of CO2 during the reaction, besides the Lewis acidic zinc center and nucleophilic halide anion.
The transformation of topological structures of N4-Zn complexes was first explored in conjunction with variable-temperature NMR experiments (Figure 2). In addition, based on studies such as NMR experiments, single crystal diffraction, and evaluation of Lewis acidity of different complexes (Figure 3), a possible reaction mechanism was proposed (Figure 4).
Relevant research was published in ACS Catalysis (https://doi.org/10.1021/acscatal.3c02449). The work has received support from the National Natural Science Foundation of China, Gansu Province of China, and the Lanzhou Institute of Chemical Physics, CAS.
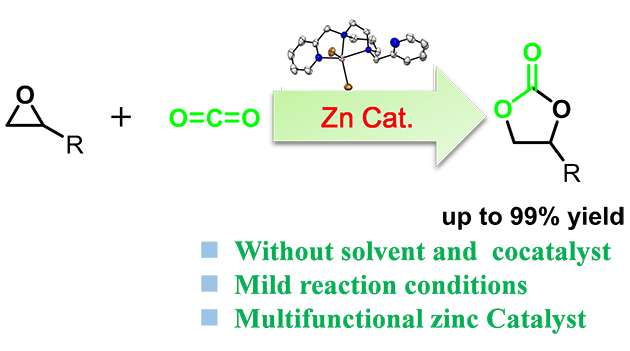
Figure 1. The cycloaddition reaction of CO2 with epoxides catalyzed by non-heme zinc complexes
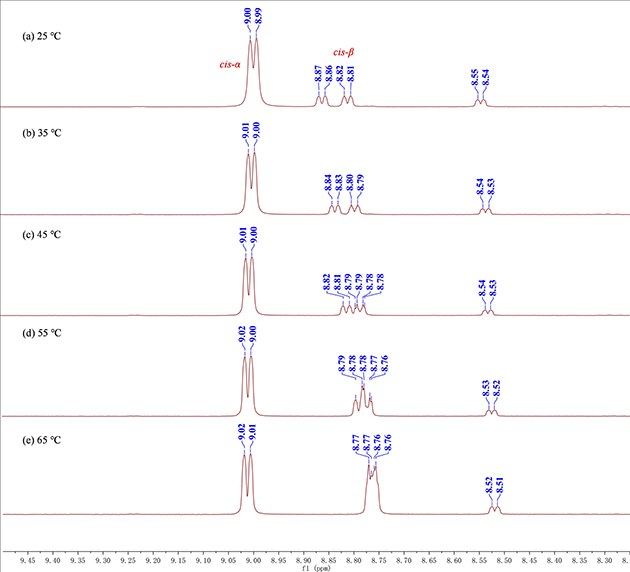
Figure 2. 1H NMR spectra of Zn-2 in dimethyl sulfoxide-d by using the variable-temperature NMR 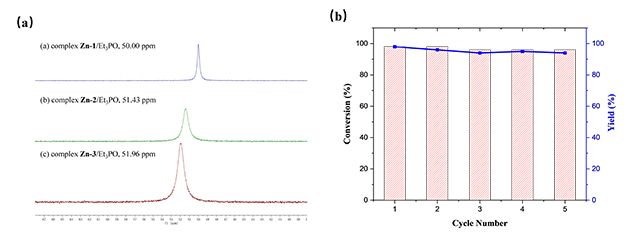
Figure 3. (a) 31P NMR spectra of complex Zn /Et3PO; (b) Recyclability of catalyst 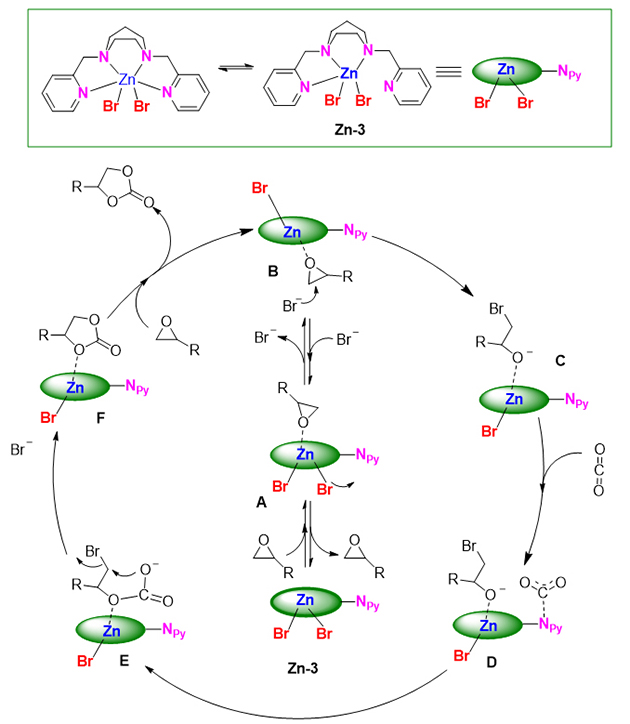
Figure 4. Proposed mechanism for the cycloaddition reaction
Contact:
Sun wei
Email: wsun@licp.cas.cn
Lanzhou Institute of Chemical Physics


