Unsymmetrical ureas can form multiple stable hydrogen bonds with proteins. Since drugs containing urea functional groups display unique biological activities when interacting with their targets, they play an important role in drug development and medicinal chemistry. As a result, finding efficient methods for synthesizing unsymmetrical ureas is important to these fields.
Current industrial synthesis of urea compounds mainly uses a three-step method involving phosgene, which generates a large amount of corrosive hydrochloric acid. Now, however, a joint research group from China has achieved one-step carbonylative synthesis of unsymmetrical urea using a non-phosgene route.
The associated study, titled "Synchronous recognition of amines in oxidative carbonylation toward unsymmetric ureas," was recently published in Science.
The catalytic oxidative carbonylation of amines is the most direct route for producing urea. However, when two different amines are used as substrates, it is difficult to differentiate them in terms of reactivity. Both symmetric and unsymmetrical ureas are generated simultaneously, making it difficult to control selectivity.
The research group, led by Prof. HE Lin of the Lanzhou Institute of Chemical Physics (LICP) of the Chinese Academy of Sciences and Prof. LEI Aiwen from Wuhan University, employed a synchronous recognition strategy that integrates a novel reaction mechanism—a nucleophilic carbonylation half-reaction (recognizing primary amines) and a radical carbonylation half-reaction (recognizing secondary amines)—into a catalytic amine oxidative carbonylation reaction.
This strategy relies on subtle differences in the physicochemical properties of different amines, enabling identification of a unique reaction window for the one-step synthesis of unsymmetrical ureas by a non-phosgene route.
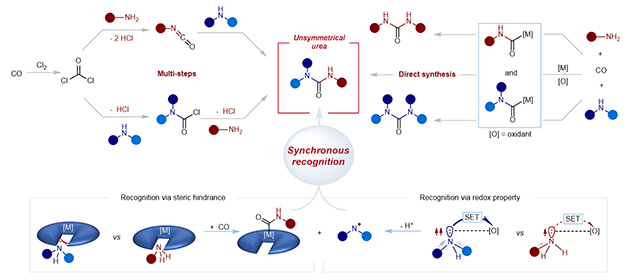
Strategies for synthesizing unsymmetrical ureas.(Image by LICP)
The new carbonylation mode is applicable not only to alkyl amines but also to various aromatic and halogenated amines.
Through catalytic carbonylation, NH3 was activated to react with secondary amines, resulting in the synthesis of corresponding unsymmetrical ureas. Intramolecular recognition of primary and secondary amines occurred as expected. Nearly one hundred combinations yielded predominantly unsymmetric products, further confirming the unique reaction mechanism.
"We can use either CO or CO2 to achieve process re-engineering for the synthesis of nitrogen-containing carbonyl compounds by the phosgene method," said Prof. HE.


