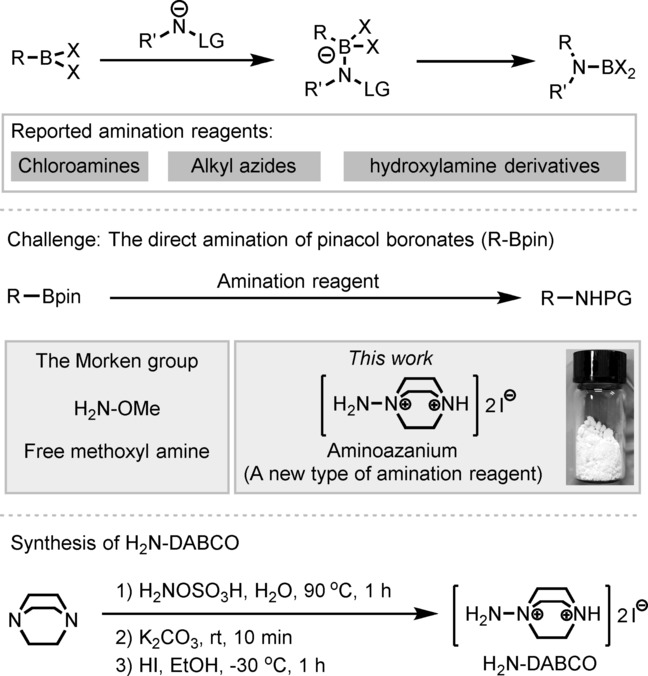
Abstract:The aminoazanium of DABCO (H2N-DABCO) has been developed as a general and practical amination reagent for the direct amination of alkyl and aryl pinacol boronates. This compound is stable and practical for use as a reagent. Various primary, secondary. and tertiary alkyl-Bpin and aryl-Bpin substrates were aminated to give the corresponding amine derivatives. The amination is stereospecific. The anti-Markovnikov hydroamination of olefins was easily achieved by catalytic hydroboration with HBpin and in subsequent situ amination using H2N-DABCO. Moreover, the combination of 1,2-diboration of olefins, using B(2)pin(2), with this amination process achieved the unprecedented 1,2-diamination of olefins. The amination protocol was also successfully extended to aryl pinacol boronates.
KeyWords Plus:BORONIC ACIDS; STEREOSPECIFIC SYNTHESIS; SECONDARY-AMINES; CHIRAL SYNTHESIS; FREE DIBORATION; ORGANIC AZIDES; METAL; ORGANOBORANES; ACCESS; ALKYLDICHLOROBORANES
Published in ANGEWANDTE CHEMIE-INTERNATIONAL EDITION,Volume: 59 Issue 7(2745-2749);10.1002/anie.201913388,FEB 10 2020


