Abstract
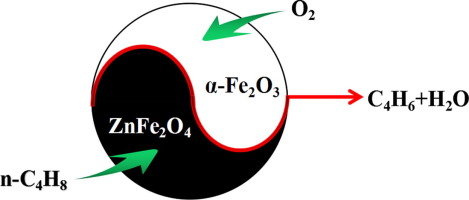
Iron-based catalysts were one of the most effective catalysts in the oxidative dehydrogenation of n-butene, however, the roles of spinel and alpha-Fe2O3 in the biphasic catalyst have remained controversial. Herein, we found that biphasic catalyst exhibited much superior catalytic performance compared to each individual component. According to the temperature programmed desorption, XPS and kinetics studies results, it was speculated that ZnFe2O4 was primarily responsible for activating n-butene and alpha-Fe2O3 was used to activate O-2. Moreover, the key factor for the catalytic activity of the biphasic catalyst were investigated and kinetic studies showed that reaction rates were well correlated with the apparent pre-exponential factor. Therefore, the efficient contact between ZnFe2O4 and alpha-Fe2O3 may lead to the improvement of the activity. In addition, the reaction mechanism over alpha-Fe2O3/ZnFe2O4 was proposed to be the interface reaction based on the catalytic evaluation results of substituting ZnFe2O4 by the redox-inactive ZnAl2O4. (C) 2019 Elsevier Inc. All rights reserved.
KeyWords Plus:IRON-OXIDE; SPINEL; BUT-1-ENE; PHASES; FE2O3; RAMAN; XPS; CO
Published in JOURNAL OF CATALYSIS,Volume 381;10.1016/j.jcat.2019.10.016,JAN 2020


