Abstract
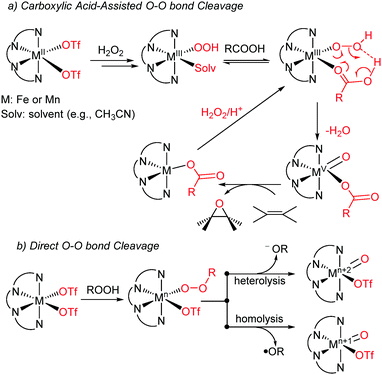
A novel manganese catalyst bearing an l-proline-derived N4 ligand has been developed for enabling acid-free asymmetric epoxidation of olefins with tert-butyl hydroperoxide as the oxidant. A variety of olefins that are well-matched in size with the ligand pocket can be transformed to epoxides with excellent enantioselectivities. The smaller ligand pocket is also beneficial to the enantioselective epoxidation of simple olefins. Cryospray ionization mass spectrometry experiments reveal that a Mn-IV?O species serves as an active epoxidizing species.
A novel manganese catalyst bearing an l-proline-derived N4 ligand has been developed for enabling acid-free asymmetric epoxidation of olefins with tert-butyl hydroperoxide as the oxidant. A variety of olefins that are well-matched in size with the ligand pocket can be transformed to epoxides with excellent enantioselectivities. The smaller ligand pocket is also beneficial to the enantioselective epoxidation of simple olefins. Cryospray ionization mass spectrometry experiments reveal that a Mn-IV?O species serves as an active epoxidizing species.
KeyWords Plus:NONHEME IRON-CATALYSTS; C-H BONDS; ASYMMETRIC EPOXIDATION; HYDROGEN-PEROXIDE; H2O2; COMPLEXES; OXIDATION
Published in CHEMICAL COMMUNICATIONS,Volume 56,13101-13104;10.1039/d0cc04440g,NOV 7 2020


