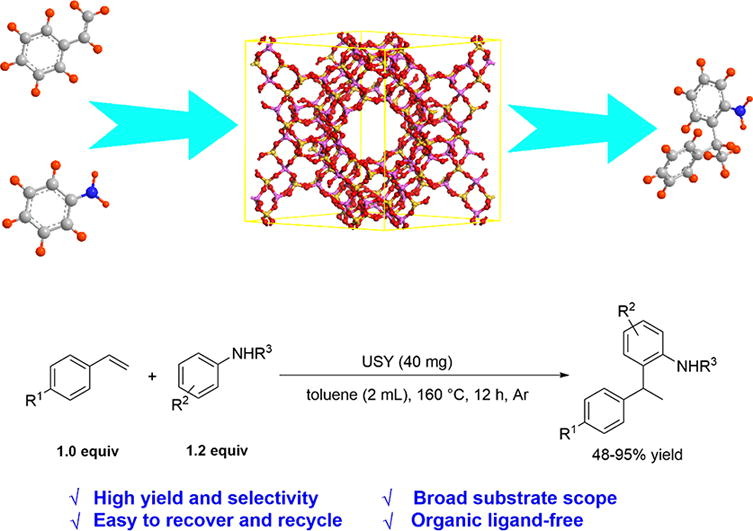
Abstract
The hydroarylation of alkenes with aromatic amines is recognized as the most atom-economical and straightforward approach to obtain functional aromatic amines, which are versatile building blocks in organic synthesis and material chemistry. However, controllable synthesis of single hydroarylation product is still a significant challenge because hydroarylation reaction often delivers four hydroarylation products and hydroamination products are also produced during the reaction. Herein, we report the first example of heterogeneous zeolite catalyzed hydroarylation of styrene and norbornene with aniline derivatives under organic ligand-free conditions. With the USY zeolite as catalyst, a wide scope of alkenes and aromatic amines with various functional groups are smoothly converted into the corresponding products in 48-95% yields with high regioselectivity. Detailed characterizations revealed that Lewis acid can promote Hofmann-Martius rearrangement of hydroamination products toward hydroarylation products, resulting in high selectivity for hydroarylation products. In addition, it could be found that the weak acid sites of zeolite play a key role in forming hydroarylation products. Furthermore, the catalyst can be reused at least 10 times without obvious deactivation. This work may promote the development of heterogeneous catalyst system for alkene hydroarylation.
Published in JOURNAL OF CATALYSIS,394;10.1016/j.jcat.2020.12.007,FEB 2021
The hydroarylation of alkenes with aromatic amines is recognized as the most atom-economical and straightforward approach to obtain functional aromatic amines, which are versatile building blocks in organic synthesis and material chemistry. However, controllable synthesis of single hydroarylation product is still a significant challenge because hydroarylation reaction often delivers four hydroarylation products and hydroamination products are also produced during the reaction. Herein, we report the first example of heterogeneous zeolite catalyzed hydroarylation of styrene and norbornene with aniline derivatives under organic ligand-free conditions. With the USY zeolite as catalyst, a wide scope of alkenes and aromatic amines with various functional groups are smoothly converted into the corresponding products in 48-95% yields with high regioselectivity. Detailed characterizations revealed that Lewis acid can promote Hofmann-Martius rearrangement of hydroamination products toward hydroarylation products, resulting in high selectivity for hydroarylation products. In addition, it could be found that the weak acid sites of zeolite play a key role in forming hydroarylation products. Furthermore, the catalyst can be reused at least 10 times without obvious deactivation. This work may promote the development of heterogeneous catalyst system for alkene hydroarylation.
Published in JOURNAL OF CATALYSIS,394;10.1016/j.jcat.2020.12.007,FEB 2021


