Abstract
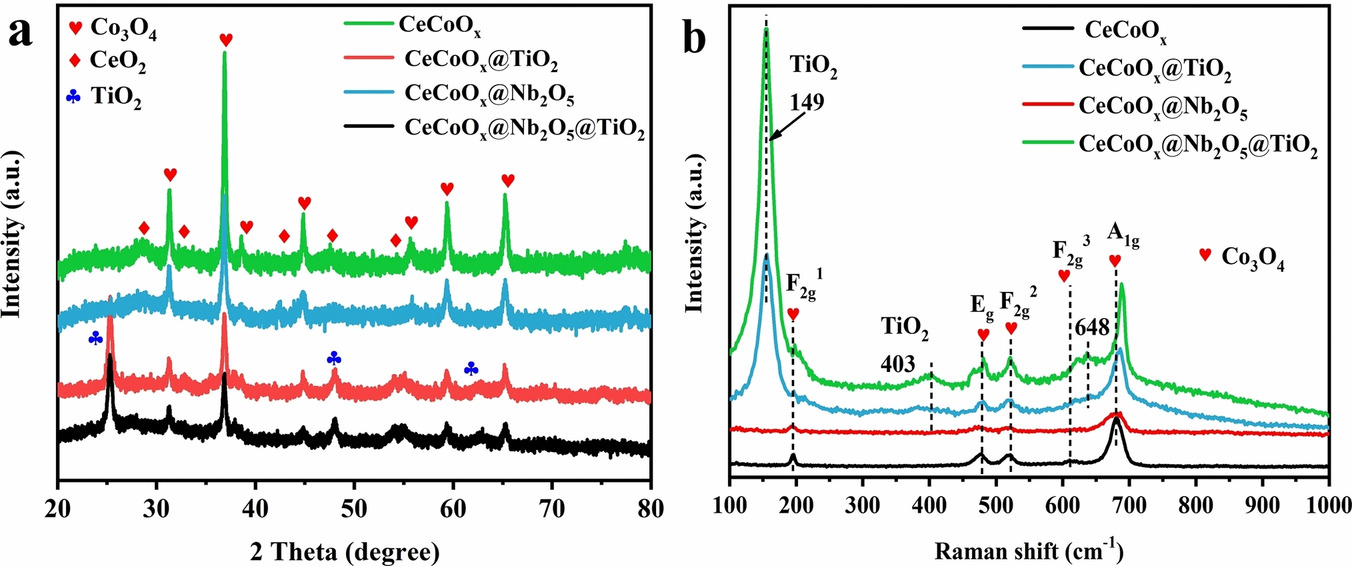
A series of catalysts with different core-shell structures has been successfully prepared by a hydrothermal method. They consisted of CeCoOx@TiO2 (single shell), CeCoOx@Nb2O5 (single shell) and CeCoOx@Nb2O5@TiO2 (double shell) core-shell nanocages and CeCoOx nanocages, in which CeCoOx was the core and TiO2 and Nb2O5 were shells. The influence of the core-shell structure on the catalytic performance of o-dichlorobenzene was investigated by activity, water-resistance, and thermal stability tests as well as catalyst characterization. The temperatures corresponding to 90% conversion of o-dichlorobenzene (T-90) of CeCoOx, CeCoOx@TiO2, CeCoOx(@)Nb(2)O(5), and CeCoOx@Nb2O5@TiO2 catalysts were 415, 383, 362 and 367 degrees C, respectively. CeCoOx@Nb2O5 exhibited excellent catalytic activity, mainly owing to the special core-shell structure, large specific surface area, abundant activity of Co3+, Ce3+, Nb5+, strong reducibility, and more active oxygen vacancies. It can be seen that the Nb2O5 coating can greatly improve the catalytic activity of the catalyst. In addition, due to the protective effect of the TiO2 shell on CeCoOx, CeCoOx@Nb2O5@TiO2 catalysts exhibited outstanding thermal and hydrothermal stability for 20 hours. The T-90 of CeCoOx@Nb2O5@TiO2 was slightly lower than that of CeCoOx@Nb2O5, but it had higher stability and hydrothermal stability. Furthermore, possible reaction pathways involving the Mars-van-Krevelen (MvK) and Langmuir-Hinshelwood (L H) models were deduced based on studies of the temperature-programmed desorption of O-2 (O-2-TPD), X-ray photoelectron spectroscopy (XPS), and in situ diffuse reflectance FTIR spectroscopy (DRIFTS) characterization.
Keywords Plus:METAL-ORGANIC FRAMEWORKLOW-TEMPERATUREMIXED OXIDESCHLORINATED AROMATICSD
Published in CHEMISTRY-A EUROPEAN JOURNAL,Volume27;10.1002/chem.202100392,JUL 16 2021


