Abstract
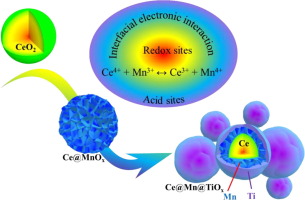
In this work, Ce@MnOx catalysts with core-shell structure were obtained via simple hydrothermal treatment, and the redox capacity can be effectively improved through co-existence of Ce and Mn. Since the strong oxidation capacity of MnOx can lead to the peroxidation of ammonia, the redox capacity can be suppressed with the decrease of manganese content. However, in the case of low manganese content, it is discovered that the surface acidity is very weak and the ammonia adsorption behavior on the catalyst is limited, which is detrimental to the NH3-SCR process. To solve this problem, a series of yolk-shell structured Ce@Mn@TiOx were accordingly constructed, where the TiO2 shell can provide more surface acid sites to promote the adsorption and activation of ammonia. As expected, the Ce@Mn@TiOx exhibits excellent catalytic performance for NH3-SCR at low temperature and possesses a relatively wide working temperature window. More importantly, the TiO2 shell could protect the active sites from water vapor competitive adsorption as well as sulfur attack. Thus, the Ce@Mn@TiOx-10% catalyst exhibited satisfied water vapor resistance in the SCR process, and its sulfur tolerance was also improved to a certain extent. In situ DRIFTs analysis demonstrated that the Ce@Mn@TiOx-10% catalyst mainly follows the L-H reaction mechanism. Consequently, the separation of acid and redox sites has been successfully accomplished by the construction of Ce@Mn@TiOx with yolk-shell structure, where the outer shell (TiO2) mainly enhanced the adsorption capacity of ammonia and protected active centers from attacking by moisture and even sulfur species, while the MnOx interlayer enhanced the interfacial electronic interaction of Ce/Mn and ensured efficient redox cycle during the NH3-SCR process.
Keywords Plus:SOLVOTHERMAL SYNTHESISSO2 RESISTANCENH3-SCRPERFORMANCEMECHANISMNBTOLERANCEPROMOTIONREMOVALACIDITY
Published in CHEMICAL ENGINEERING JOURNAL,Volume 419;10.1016/j.cej.2021.129572,SEP 1 2021


