Abstract
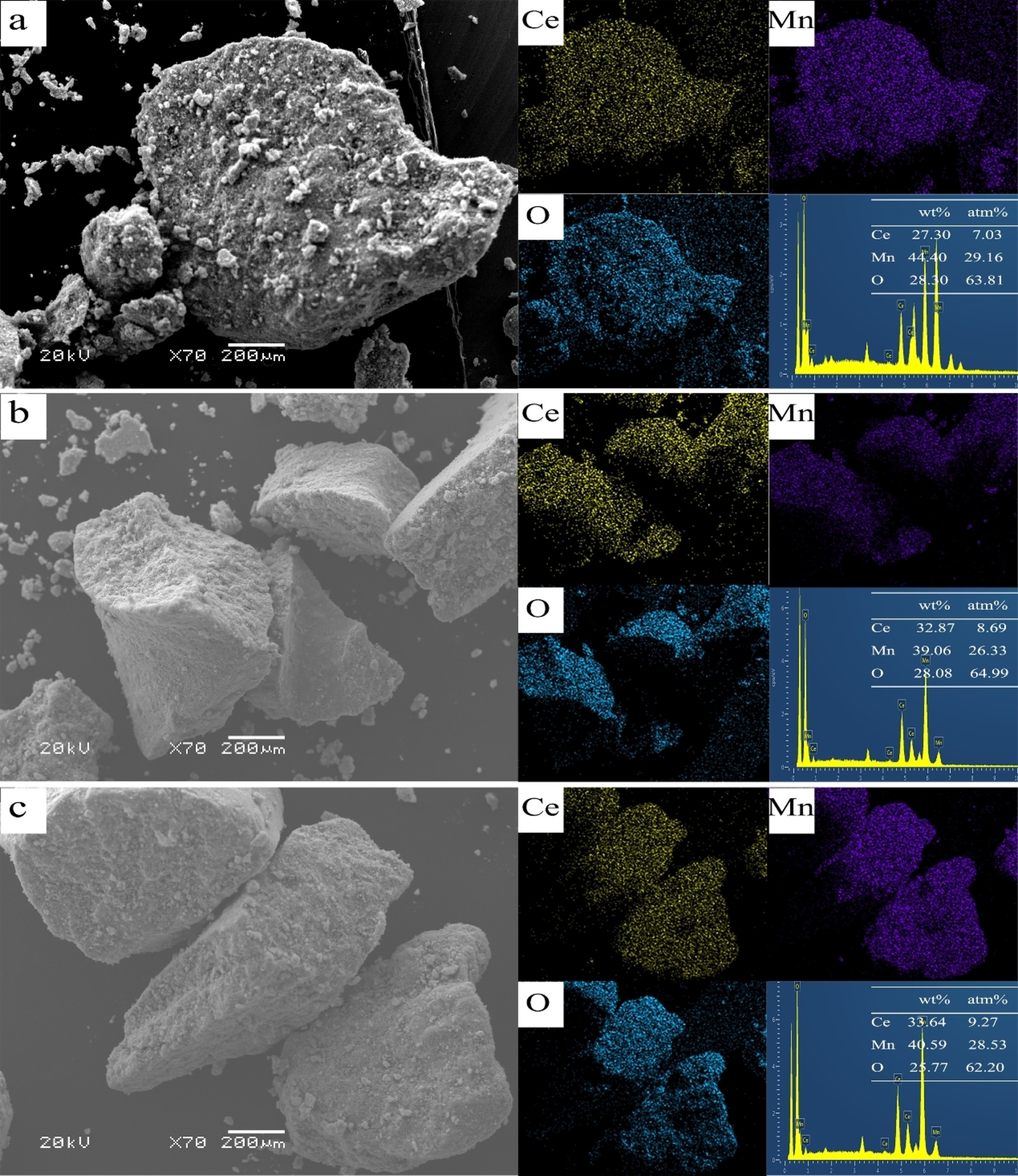
Ce(1)Mn(3)Ox with broccoli morphology were synthesized by simple redox-precipitation. A series of Ce-Mn-based catalysts with different activities were obtained by changing calcination time. The catalytic activity of Ce-Mn solid solution was greatly improved compared with that of pure oxides. Ce(1)Mn(3)Ox-1 (C1M3-1) briefly calcined at 400 degrees C showed the optimal catalytic performance (WHSV=60000 mL h(-1) g(-1), Toluene concentration=3000 ppm, T(100%=)180 degrees C). The characterization results unambiguously identified that C1M3-1 had a large amount of Ce3+, Mn3+ and surface adsorbed oxygen. This work emphasized the preparation of Ce-Mn-based solid solution with strong interaction by simple redox precipitation, and the activity of the catalyst was regulated by changing the calcination time. The advantages of low cost, high efficiency, good stability, and recycling indicated that the catalyst had the possibility of industrial application.
Keywords Plus:VOLATILE ORGANIC-COMPOUNDSOXYGEN VACANCIESOXIDATIONPERFORMANCEOXIDESCHLOROBENZENEREMOVALMNO2BENZENEVOCS
Published in CHEMCATCHEM,10.1002/cctc.202100974,AccessAUG 2021


