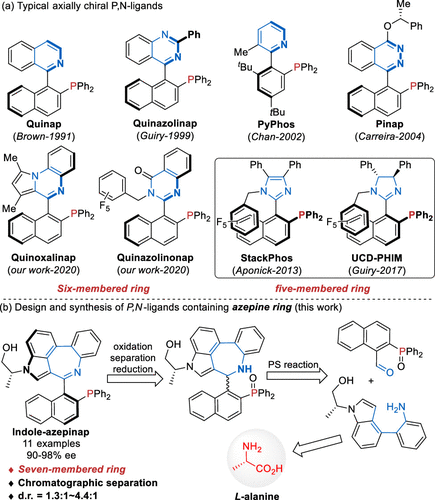
Abstract
A family of axially chiral azepine-containing seven-membered cyclic P,N-ligands (named Indole-azepinap) have been prepared by using L-alanine as an original chirality source. The direct chromatographic separation of two diastereomeric phosphine oxides on silica gel enabled these ligands to be easy available, allowing further structural and electronic modifications. Preliminary application of these Indole-azepinaps has been demonstrated in a Pd-catalyzed asymmetric allylic alkylation with high yields and moderate enantioselectivities.
Keywords Plus:CATALYTIC ASYMMETRIC-SYNTHESISPICTET-SPENGLER REACTIONKINETIC RESOLUTIONRHODIUM COMPLEXESBRONSTED ACIDSLIGANDSHYDROBORATIONACCESSQUINAP
Published in ORGANIC LETTERS,Volume 23;10.1021/acs.orglett.1c02834,OCT 15 2021


