Abstract
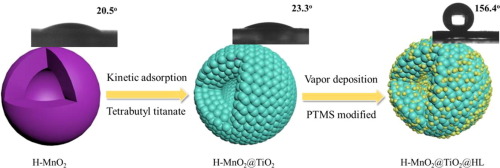
The Mn-based catalyst with hollow spherical structure was prepared by hydrothermal method and used for selective catalytic reduction (SCR) of NO with NH3. Mn-based catalysts exhibit excellent de-NOx performance at low-temperature, but narrow operating temperature window and poor water resistance restrict its application. Effectively expanding the operating temperature window and improving the H2O resistance of the Mn-based catalyst are the keys to industrial application. In this article, the H-MnO2 catalyst was prepared by hydrothermal method and modified by kinetic adsorption and vapor deposition methods. The TiO2 layer can effectively limit the excessive redox performance and improve the acidity of the catalyst surface, the NO conversion above 90% at 140-380 degrees C of H-MnO2@TiO2 catalyst. The phenyltrimethoxysilane was deposited on the surface of the H-MnO2@TiO2 catalyst by vapor deposition method. The NO conversion rate of the H-MnO2@TiO2@HL catalyst exceeds 80% in the temperature range of 180-380 degrees C. When the H2O was introduced, the NO conversion rate increases from 70% to 85% at 180 degrees C. The in situ DRIFTs results showed NH3 and NO could be adsorbed and activated on the catalyst surface, and the catalytic reaction follows Langmuir-Hinshelwood (L-H) mechanism. Under the presence of H2O, the NH4 bonding to Bronsted acid sites were inhibited but the NH3 bonding to Lewis acid site was almost unaffected. In summary, through two-step modification, the operating temperature window and water resistance of Mn-based catalysts are effectively improved. It can be seen that a reasonable structure design can effectively improve the comprehensive performance of the catalyst.
Keywords Plus:LOW-TEMPERATURE NH3-SCRMIXED-OXIDE CATALYSTNO REDUCTIONNITRIC-OXIDENH3OXIDATIONPERFORMANCEMECHANISMAMMONIACERIA
Published in CHEMICAL ENGINEERING JOURNAL,Volume 426;10.1016/j.cej.2021.131334,DEC 15 2021


