Abstract
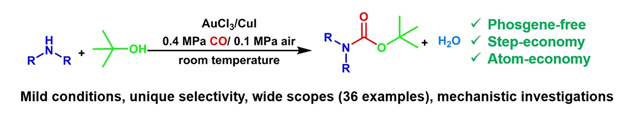
N-tert-butoxycarbonyl (N-Boc) amines are useful intermediates in synthetic/medicinal chemistry. Traditionally, they are prepared via an indirect phosgene route with poor atom economy. Herein, a step- and atom-economic synthesis of N-Boc amines from amines, t-butanol, and CO was reported at room temperature. Notably, this N-tert-butyloxycarbonylation procedure utilized ready-made substrates, commercially available AuCl3/CuI as catalysts, and O-2 from air as the sole oxidant. This catalytic system provided unique selectivity for N-Boc amines in good yields. More significantly, gram-scale preparation of medicinally important N-Boc amine intermediates was successfully implement, which demonstrated a potential application prospect in industrial syntheses. Furthermore, this approach also showed good compatibility with tertiary and other useful alcohols. Investigations of the mechanisms revealed that gold catalyzed the reaction and copper acted as electron transfer mediator in the catalytic cycle.
Keywords Plus:CATALYZED OXIDATIVE CARBONYLATIONIONIC-LIQUIDEFFICIENTPALLADIUMPROTECTIONALCOHOLSACIDGOLDCOMPLEXESRESOLUTION
Published in CHEMSUSCHEM;10.1002/cssc.202102400,DEC 2021


