Abstract
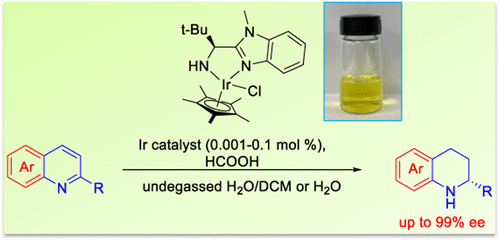
An asymmetric transfer hydrogenation (ATH) of quinolines in water or biphasic systems was developed. This ATH reaction proceeds smoothly without the need for inert atmosphere protection in the presence of a water-soluble iridium catalyst, which bears an easily available aminobenzimidazole ligand. This ATH system can work at a catalyst loading of 0.001 mol % (S/C = 100 000, turnover number (TON) of up to 33 000) under mild reaction conditions. The turnover frequency (TOF) value can reach as high as 90 000 h(-1). A variety of quinoline and N-heteroaryl compounds are transformed into the desired products in high yield and up to 99% enantiomeric excess (ee).
Keywords Plus:HIGHLY ENANTIOSELECTIVE HYDROGENATIONMETAL-FREE HYDROGENATIONSKINETIC RESOLUTIONFORMIC-ACIDAIRCHEMISTRYALDEHYDESROBUSTENHANCEMENTDERIVATIVES
Published in JOURNAL OF ORGANIC CHEMISTRY,Volume86;10.1021/acs.joc.1c01925,DEC 3 2021


