Abstract
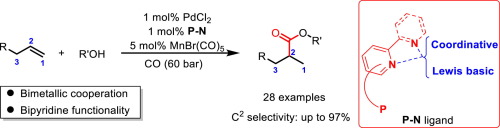
Carbazole derived phosphine ligands containing pyridine moiety were designed and prepared. It allows the challenging Pd-catalyzed Markovnikov-selective alkoxycarbonylation of aliphatic alkenes to give the branched ester products (28 examples; 51-97% branch selectivity). Preliminary mechanistic studies sup-port that the bridging bromide between Pd and Mn centers is crucial for the enhancement of regioselectivity. (c) 2022 Elsevier Inc. All rights reserved.
Keywords Plus:PALLADIUM-CATALYZED ALKOXYCARBONYLATIONBIMETALLIC CATALYSISCARBONYLATIONCOMPLEXESMETHOXYCARBONYLATIONHYDROXYCARBONYLATIONMECHANISMHYDROESTERIFICATIONSTYRENEREGIOSELECTIVITY
Published in JOURNAL OF CATALYSIS,Volume409;10.1016/j.jcat.2022.03.029,MAY 2022


