Abstract
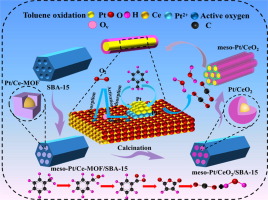
In this study, the confined Pt/Ce-MOF were for the first time synthesized using an in-situ vacuum self assembly strategy. It is confirmed that the meso-Pt/CeO2 catalyst derived from the confined Pt/Ce-MOF has excellent activity and stability in the catalytic oxidation of toluene, which is mainly due to the effective anchoring of Pt nanoparticles in Ce-MOF, and the interface effect between Pt nanoparticles and CeO2. More importantly, it is discovered that the double-layer confinement effect of ordered mesoporous SBA 15 and Ce-MOF can effectively avoid the migration and aggregation of Pt nanoparticles, making meso-Pt/ CeO2 catalysts exhibit higher Pt dispersion. The structure-activity relationship and reaction mechanism of the meso-Pt/CeO2 catalyst were explored by in situ surface-enhanced Raman spectroscopy (SERS) and in situ DRIFTs spectra. The results show that oxygen molecules in the air are firstly adsorbed to the surface of the catalyst, and then are activated as Ce-O and Pt-O active species during the reaction process. The benzyl alcohol, benzoic acid, benzoate and maleic anhydride are the main intermediate products, indicating that the reaction pathway may be as follows: adsorption of toluene-. benzyl alcohol-. benzoic acid-. benzoate-. maleic anhydride-. ...-. carbon dioxide and water. Simultaneously, it is verified that the benzyl alcohol (C6H5-CH2O-) and carboxylic acid species is the decisive step in the oxidation of toluene on basis of in situ SERS and DRIFTs spectra.(c) 2022 Elsevier Inc. All rights reserved.
In this study, the confined Pt/Ce-MOF were for the first time synthesized using an in-situ vacuum self assembly strategy. It is confirmed that the meso-Pt/CeO2 catalyst derived from the confined Pt/Ce-MOF has excellent activity and stability in the catalytic oxidation of toluene, which is mainly due to the effective anchoring of Pt nanoparticles in Ce-MOF, and the interface effect between Pt nanoparticles and CeO2. More importantly, it is discovered that the double-layer confinement effect of ordered mesoporous SBA 15 and Ce-MOF can effectively avoid the migration and aggregation of Pt nanoparticles, making meso-Pt/ CeO2 catalysts exhibit higher Pt dispersion. The structure-activity relationship and reaction mechanism of the meso-Pt/CeO2 catalyst were explored by in situ surface-enhanced Raman spectroscopy (SERS) and in situ DRIFTs spectra. The results show that oxygen molecules in the air are firstly adsorbed to the surface of the catalyst, and then are activated as Ce-O and Pt-O active species during the reaction process. The benzyl alcohol, benzoic acid, benzoate and maleic anhydride are the main intermediate products, indicating that the reaction pathway may be as follows: adsorption of toluene-. benzyl alcohol-. benzoic acid-. benzoate-. maleic anhydride-. ...-. carbon dioxide and water. Simultaneously, it is verified that the benzyl alcohol (C6H5-CH2O-) and carboxylic acid species is the decisive step in the oxidation of toluene on basis of in situ SERS and DRIFTs spectra.(c) 2022 Elsevier Inc. All rights reserved.
Keywords Plus:METAL-ORGANIC FRAMEWORKSIN-SITU PYROLYSISTOLUENE COOXIDATIONMETHANE OXIDATIONHIGH-PERFORMANCEOXIDE CATALYSTSCOMBUSTIONINSIGHTSCOSINGLE
Published in JOURNAL OF CATALYSIS,Volume412;10.1016/j.jcat.2022.05.022,AUG 2022


