Abstract
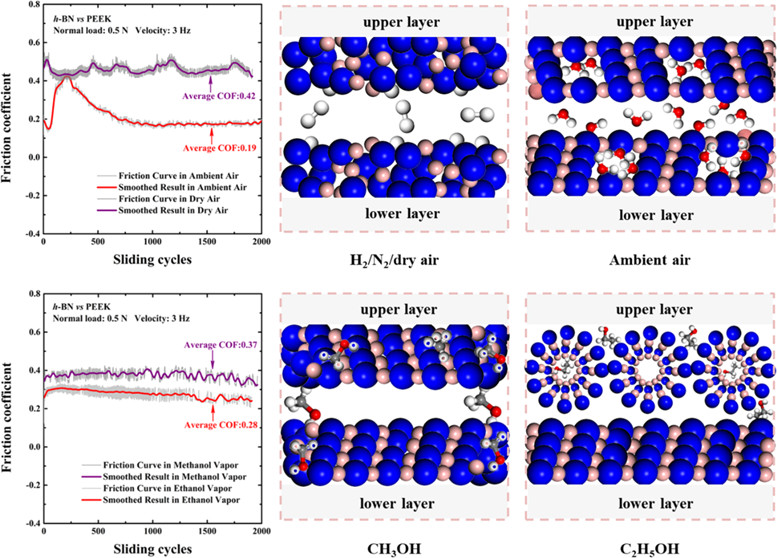
Sliding contact experiments and first-principles calculations were performed to elucidate the roles of environmental molecules containing -OH functional groups on the friction behavior and structural evolution of hexagonal boron nitride (h-BN). A significant decrease in the friction coefficient (COF) is established by the physisorption and dissociative adsorption of molecules containing -OH functional groups on h-BN, compared with that in a H-2 or N-2 atmosphere. A key finding is the existence of two friction mechanisms to reconstruct the sliding interface for h-BN crystallites in humid air and carbon contaminant (CH3OH and C2H5OH) atmospheres, which is verified by the friction behavior and morphologies of the wear track. There is a running-in period in the friction process to induce the formation of defects in h-BN in humid air, which facilitates dissociative adsorption of water molecules on h-BN. The formation of nanostructured water at defect sites will promote lamellar slip of h-BN crystal materials for friction reduction. In carbon contaminant environments, both molecules exhibit strong adsorption on the h-BN surface regardless of the presence of defects, thereby weakening the structural damage rate and enhancing the bearing capacity. C2H5OH molecules are more likely to dissociate and bind onto defect sites, endowing h-BN with high in-plane stress to form a coiled structure. h-BN in the annular or tubular form would exhibit a self-protective effect, facilitate incommensurate contact, and reduce the contact area to enhance lubrication. Our work may establish the fundamental basis for future applications of h-BN in new energy vehicles.
Sliding contact experiments and first-principles calculations were performed to elucidate the roles of environmental molecules containing -OH functional groups on the friction behavior and structural evolution of hexagonal boron nitride (h-BN). A significant decrease in the friction coefficient (COF) is established by the physisorption and dissociative adsorption of molecules containing -OH functional groups on h-BN, compared with that in a H-2 or N-2 atmosphere. A key finding is the existence of two friction mechanisms to reconstruct the sliding interface for h-BN crystallites in humid air and carbon contaminant (CH3OH and C2H5OH) atmospheres, which is verified by the friction behavior and morphologies of the wear track. There is a running-in period in the friction process to induce the formation of defects in h-BN in humid air, which facilitates dissociative adsorption of water molecules on h-BN. The formation of nanostructured water at defect sites will promote lamellar slip of h-BN crystal materials for friction reduction. In carbon contaminant environments, both molecules exhibit strong adsorption on the h-BN surface regardless of the presence of defects, thereby weakening the structural damage rate and enhancing the bearing capacity. C2H5OH molecules are more likely to dissociate and bind onto defect sites, endowing h-BN with high in-plane stress to form a coiled structure. h-BN in the annular or tubular form would exhibit a self-protective effect, facilitate incommensurate contact, and reduce the contact area to enhance lubrication. Our work may establish the fundamental basis for future applications of h-BN in new energy vehicles.
Keywords Plus:GENERALIZED GRADIENT APPROXIMATIONWEAR-RESISTANCESURFACESETHANOL
ACS APPLIED MATERIALS & INTERFACES
Published in Volume14;10.1021/acsami.2c02450,APR 27 2022


