Abstract
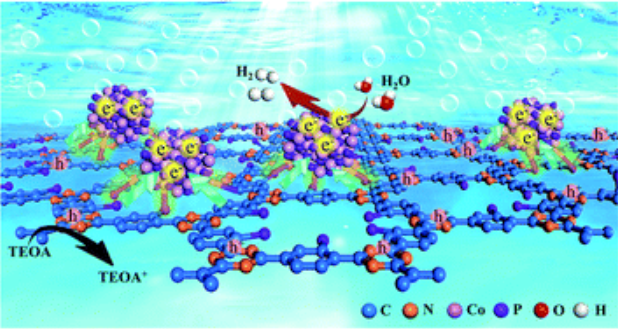
Covalent triazine-based frameworks (CTFs) have emerged as some of the most important materials for photocatalytic water splitting. However, development of CTF-based photocatalytic systems with non-platinum cocatalysts for highly efficient hydrogen evolution still remains a challenge. Herein, we demonstrated, for the first time, a one-step phosphidation strategy for simultaneously achieving phosphorus atom bonding with the benzene rings of CTFs and the anchoring of well-defined dicobalt phosphide (Co2P) nanocrystals (similar to 7 nm). The hydrogen evolution activities of CTFs were significantly enhanced under simulated solar-light (7.6 mmol h(-1) g(-1)), more than 20 times higher than that of the CTF/Co2P composite. Both comparative experiments and in situ X-ray photoelectron spectroscopy reveal that the strong interfacial P-C bonding and the anchoring of the Co2P cocatalyst reverse the charge transfer direction from triazine to benzene rings, promote charge separation, and accelerate hydrogen evolution. Thus, the rational anchoring of transition-metal phosphides on conjugated polymers should be a promising approach for developing highly efficient photocatalysts for hydrogen evolution.
Keywords Plus:NANOPARTICLESNANOSHEETSCATALYSTSG-C3N4CO2P
Published in CHEMICAL SCIENCE,Volume13;10.1039/d2sc02638d,JUL 13 2022


