Abstract
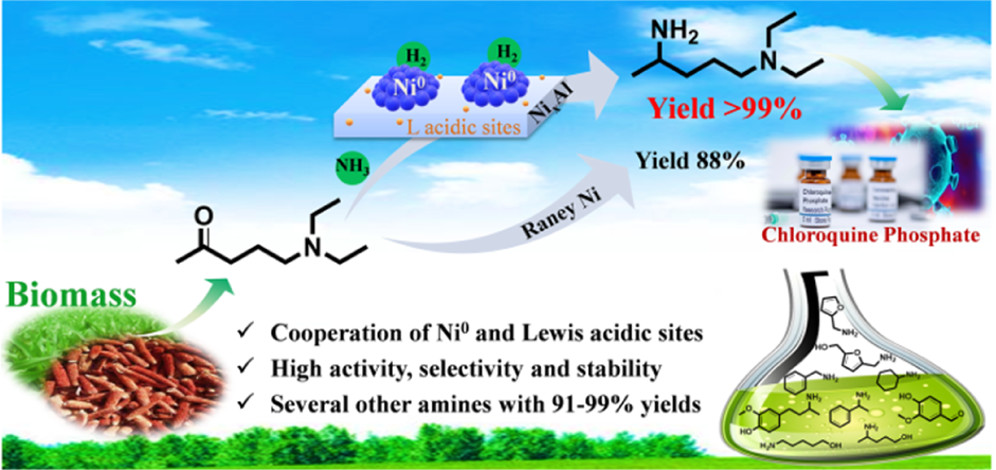
Heterogeneous catalytic reductive amination of biomass-based carbonyl compounds is a highly attractive strategy to realize green and sustainable amine synthesis under mild conditions. Herein, N-1, N-1-diethyl-1,4-pentanediamine, a key side chain of chloroquine pharmaceuticals, was highly selectively synthesized through the reductive amination of 5-diethylamino-2-pentanone on active NixAl nanocatalysts with different Ni/Al molar ratios prepared by the simple co-precipitation method. The Ni1Al catalyst presented the highest target diamine product yield (up to 99%) among the NixAl catalysts. This catalyst also exhibited remarkable stability in a fixed-bed reactor without deactivation after 200 h of continuous operation, thanks to its good anti-sintering and anti-oxidation properties. Systematic structure characterizations and catalytic activity tests showed that their catalytic activity depended sensitively on the Ni/Al molar ratio, which governed the dispersion, reducibility, and surface acidity of the catalysts. The synergic catalysis of highly dispersed active Ni-0 nanoparticles with abundant adjacent surface Lewis acidic sites accounted for the outstanding catalytic performance of the Ni1Al catalyst. Moreover, such novel catalysts also presented high activity in the reductive amination of several other biomass-derived aldehydes and ketones to produce the corresponding amines, achieving 91-99% yields under mild conditions of 80-100 degrees C and 1-2 MPa.
Heterogeneous catalytic reductive amination of biomass-based carbonyl compounds is a highly attractive strategy to realize green and sustainable amine synthesis under mild conditions. Herein, N-1, N-1-diethyl-1,4-pentanediamine, a key side chain of chloroquine pharmaceuticals, was highly selectively synthesized through the reductive amination of 5-diethylamino-2-pentanone on active NixAl nanocatalysts with different Ni/Al molar ratios prepared by the simple co-precipitation method. The Ni1Al catalyst presented the highest target diamine product yield (up to 99%) among the NixAl catalysts. This catalyst also exhibited remarkable stability in a fixed-bed reactor without deactivation after 200 h of continuous operation, thanks to its good anti-sintering and anti-oxidation properties. Systematic structure characterizations and catalytic activity tests showed that their catalytic activity depended sensitively on the Ni/Al molar ratio, which governed the dispersion, reducibility, and surface acidity of the catalysts. The synergic catalysis of highly dispersed active Ni-0 nanoparticles with abundant adjacent surface Lewis acidic sites accounted for the outstanding catalytic performance of the Ni1Al catalyst. Moreover, such novel catalysts also presented high activity in the reductive amination of several other biomass-derived aldehydes and ketones to produce the corresponding amines, achieving 91-99% yields under mild conditions of 80-100 degrees C and 1-2 MPa.
Keywords Plus:REDUCTIVE AMINATIONSELECTIVE SYNTHESISALCOHOL AMINATIONGENERAL-SYNTHESISPRIMARY AMINESCATALYSTSMETHANECHEMICALSCONVERSIONOXIDATION
Published in ACS SUSTAINABLE CHEMISTRY & ENGINEERING;10.1021/acssuschemeng.2c00115,APR 2022


