Abstract
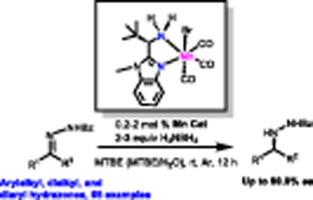
The enantioselective reduction of C=N bonds constitutes an effective strategy for the production of chiral amines. Herein, we report the first example of manganese-catalyzed asymmetric transfer hydrogenation of hydrazones by employing a readily available chiral aminobenzimidazole manganese(I) complex under mild conditions. The present protocol allows for the enantioselective transfer hydrogenation of a wide range of arylalkyl, dialkyl and diaryl hydrazones, providing the desired chiral hydrazines in excellent yields and enantioselectivities (65 examples, up to 99.9% ee). Of note, the current method is compatible with the challenging diaryl hydrazones without the requirement of an ortho-substitution on the phenyl ring. A preliminary study of the mechanism suggests that a manganese-hydride pathway is involved, and the high enantiocontrol of the reaction is attributed to a it-it stacking interaction between substrate and catalyst. (C) 2022 Elsevier Inc. All rights reserved.
Keywords Plus:ENANTIOSELECTIVE HYDROGENATIONTRANSITION-METALREDUCTIVE AMINATIONPHOSPHORUS LIGANDSKETONESIRONIMINESAMINESCOBALTCOMPLEXES
Published in JOURNAL OF CATALYSIS,Volume413;10.1016/j.jcat.2022.06.045,SEP 2022


