Abstract
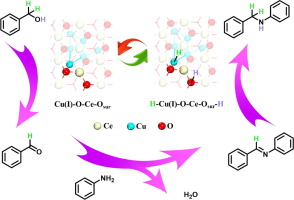
The interface between metal sites and metal oxides sometimes acts as the real active site, but the iden-tification of its structure and function in catalysis is still elusive. Here, we demonstrate that Lewis acid-base pairs (LPs) at the interface of Cu/CeO2 catalyst display outstanding catalytic performance on the hydrogen borrowing reaction under base free conditions. Characterizations disclose that LPs consist of Lewis acidic Cu(I) and Lewis basic surface oxygen (Osurf), where the Cu(I) species are formed by electron transfer between the interfacial Cu and Ce {e.g., Ce(III) + Cu(II) M Ce(IV) + Cu(I)} and Osurf species are simultaneously created by surface oxygen mobility. The Cu(I)-H and Osurf-H intermediates are generated by reacting hydride (H-) and proton (H+) from C-H and O-H cleavage with Lewis acidic Cu(I) and Lewis basic Osurf, respectively. The combination of control experiments, deuterium isotope studies and DFT cal-culations reveals that the Cu(I)-H and Osurf-H intermediates are spatially isolated by LPs and prefer to transfer to C@N bonds rather than forming H2 molecules, leading to outstanding selectivity towards the desired N-alkyl amine.(c) 2023 Elsevier Inc. All rights reserved.
Keywords Plus:TOTAL-ENERGY CALCULATIONSSELECTIVE N-METHYLATIONHETEROGENEOUS CATALYSTSPHASE RELATIONSHIPSSURFACE-AREAMIXED OXIDESIN-SITUEFFICIENTALKYLATIONNANORODS
Published in JOURNAL OF CATALYSIS,Volume418;10.1016/j.jcat.2023.01.020,FEB 2023


