Abstract
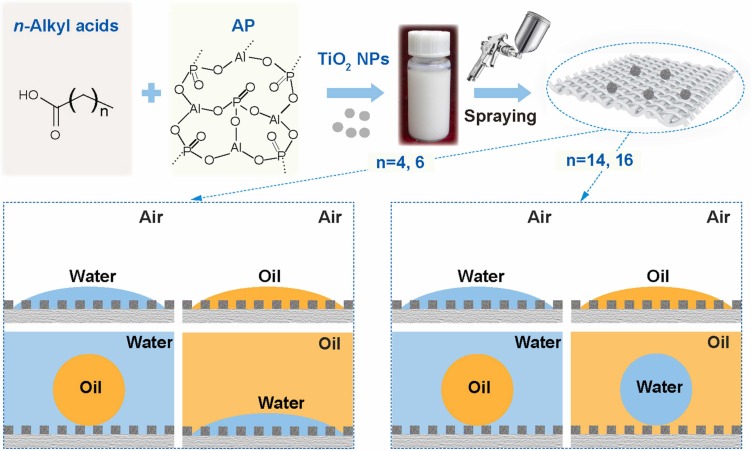
Superwetting that is being subdivided to multiple superlyphobic states exists unique superiority in a specific interfacial application. Fine controlling superwetting surface is lack of systematic research. Due to the harsh conditions on the creation of underoil superhydrophilicity and suerhydrophobicity in oil of underwater superoleophobic surface, so far controllable fabrication strategies of the underwater superoleophobic surfaces in oil still remain a challenge. Herein, underwater superoleophobic surfaces from superhydrophilicity to superhydrophobicity in oil are controllable constructed by mixing commercial titanium dioxide nanoparticles, aluminum phosphate, and n-Alkyl acids, where the surface heterogeneous chemistry is finely controlled by changing the chain length of n-alkanoic acids. Under the rational surface energy, underoil superhydrophilicity and underoil superhydrophobicity of the underwater superoleophobic surfaces can be efficiently regulated via simply adjusting the chain length of n-alkanoic acids. The under-liquid dual superlyophobic surface can be achieved when an appropriate parameter denoted chain length of n-Alkyl acids (n) is between 14 and 16. However, when the parameter (n) is to be within 4 and 6, the superhydrophilicity in oil of underwater superoleophobic surface can be realized. This finding can connect the molecular-level conformational transformation and the conversion of macroscopic surface alternative wettability, providing the molecular-level design principle of superwetting interfacial in under-liquid environments.
Keywords Plus:SEPARATION
Published in TRIBOLOGY INTERNATIONAL,Volume183;10.1016/j.triboint.2023.108427,MAY 2023


