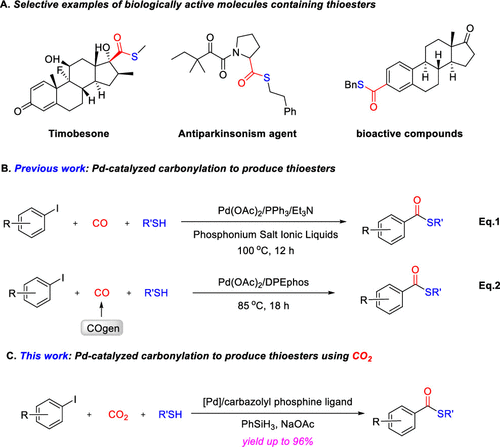
Abstract
The first example of catalytic thiocarbonylation of aryliodidesusing CO2 has been achieved employing a combination ofPdCl(2) and carbazole-derived phosphine ligands. Under mildconditions, a broad scope of aryl iodides were converted to the desiredthioester products in the presence of aryl or alkyl thiols (33 examples,up to 96% yields). The choice of metal, ligands, and reductant werecrucial for high efficiency and chemoselectivity. Moreover, this strategyprovided an effective method for the late-stage functionalizationof biorelevant molecules.
Keywords Plus:CARBON-MONOXIDECARBOXYLIC-ACIDSBUILDING-BLOCKTHIOESTERSTHIOLSTHIOESTERIFICATIONCARBONYLATIONESTERIFICATIONALDEHYDESFORMYLATION
Published in JOURNAL OF ORGANIC CHEMISTRY;10.1021/acs.joc.3c00643,MAY 2023


