Abstract
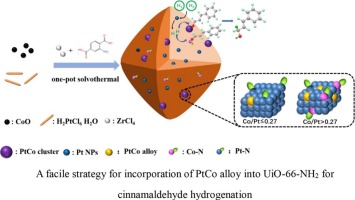
As a material with multifunctional ligands, metal-organic frameworks (MOFs) can effectively regulate the electronic and geometric state of supported metal nanoparticles (MNPs). Herein, a strategy of PtCo alloy incorporated into UiO-66-NH2 hybrid nanoparticles by a facile one-pot solvothermal method was developed. A series of PtCoy@UiO-66-NH2 materials with different Co/Pt ratios were successfully synthesized using CoO as the cobalt source. The obtained PtCo0.80@UiO-66-NH2 exhibited excellent conversion (94.3%) with high selectivity (93.3%) for selective hydrogenation of cinnamaldehyde (CAL) to cinnamyl alcohol (COL). Moreover, the formation rate of COL achieved to 5 mol center dot g(Pt)(-1)center dot h(-1) as Co/Pt atomic ratio was higher than 0.41. The synergistic effect of Pt, Co and UiO-66-NH2 was investigated by various characterizations. The results revealed that the Lewis acid CoNx favored the linear adsorption of C=O bond, and PtCo alloy enhanced activity and selectivity by lowering the activation barrier for C=O bond activation and simultaneously suppressing adsorption of C=C bond.
Keywords Plus:METAL-ORGANIC FRAMEWORKSSELECTIVE HYDROGENATIONBIMETALLIC CATALYSTSCOPLATINUMNANOPARTICLEADSORPTIONNANOCLUSTERSPALLADIUMEVOLUTION
Published in CATALYSIS COMMUNICATIONS,Volume181,10.1016/j.catcom.2023.106714;AUG 2023


