Abstract
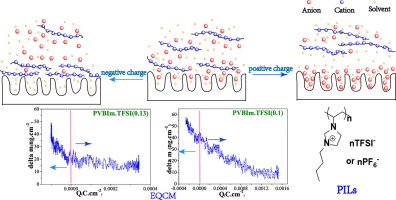
A fundamental understanding of the charge storage mechanism of electrochemical double-layer capacitors (EDLCs) requires an in-depth storage behavior investigation of independent ions (individual cations or anions) on the electrode surface. The direct in-situ observation for energy storage behavior of individual ions under realistic conditions remains challenge. Herein, in-situ monitoring the energy storage behavior of independent ions is realized with assistance of polymeric ionic liquids skillfully. Triangle cyclic voltammogram (CV) curves indicate that polymeric imidazole cations PVBIm+ with huge size cannot enter the active carbon pores upon charging, whereas only free anions can. The CV and electrochemical quartz crystal microbalance (EQCM) simultaneously detect ion adsorption/desorption during charging. It's proposed that ion adsorption/desorption is governed by electrostatic attraction and repulsion of charged particles. PVBIm+ are adsorbed on the electrode surface due to ion adsorption mechanism during the negative polarization and have a small capacity contribution. Free anions contribute capacitance, their number entering the pore is determined by the interaction between polycaions and anions. Moreover, PVBIm(0.1)+ with higher polymerization degree may promote desorption of polycations and prevent further adsorption of anions at higher potential in positive polarization. This work not only observes the charging behavior of independent ions, but also reveals the relevant charging mechanism through EQCM, paving the way for panorama of EDLC.
Keywords Plus:DIFFUSION,DYNAMICS,SIZE,PERFORMANCE,MIXTURES
Published in CHEMICAL ENGINEERING JOURNAL,Volume460,10.1016/j.cej.2023.141704;MAR 15 2023


