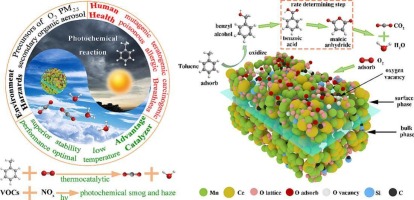
Abstract
Trace activated carbon (AC) and diatomaceous earth (DE) were used as structural promoters to be incorporated into Ce-Mn-based solid-solution catalysts by the redox precipitation method. The modified catalysts exhibit superior reducibility, with abundant Ce3+, Mn3+and reactive oxygen species, which are facilitated to the migration of oxygen and the generation of oxygen vacancies. In particular, the catalytic combustion temperatures of 90 % toluene (3000 ppm) on Ce1Mn3Ox-AC/DE were 84 degrees C (dry) and 123 degrees C (10 vol% H2O), respectively. The role of lattice oxygen and adsorbed oxygen was revealed by in situ DRIFTS. Additionally, in situ DRIFTS was employed to verify that the degradation of toluene by Ce1Mn3Ox-AC/DE satisfied the LangmuirHinshelwood (L-H) mechanism and the Mars-Van Krevelen (MvK) mechanism. The possible reaction pathway was elucidated (toluene -> benzyl alcohol -> benzoic acid -> maleic anhydride -> CO2 + H2O). Furthermore, final products attributed to toluene oxidation were detected by in situ DRIFTS at 50 degrees C in the absence of oxygen, confirming that the catalyst possessed outstanding performance at low temperatures beyond mere adsorption.
Keywords Plus:MANGANESE OXIDE,GASEOUS BENZENE,LATTICE OXYGEN,OXIDATION,PERFORMANCE,CO,FORMALDEHYDE,CHLOROBENZENE,NH3-SCR,VOCS
Published in SCIENCE OF THE TOTAL ENVIRONMENT,Volume912,10.1016/j.scitotenv.2023.169192;FEB 20 2024


