Abstract
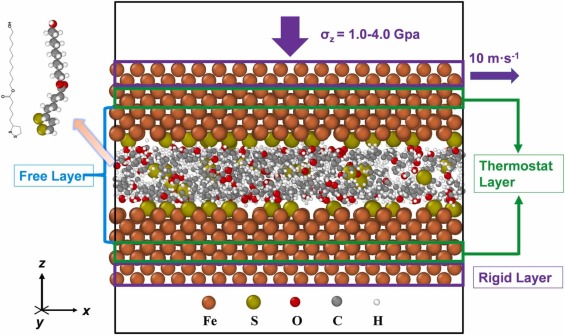
Tribofilm formation and growth play a vital role in friction reduction and antiwear enhancement. However, our understanding at the atomic level, particularly from a tribochemical viewpoint, is limited. Here, we elucidate alpha-lipoic acid ester tribofilm formation on ferrous interfaces using reactive molecular dynamics simulations. Our findings uncover a sequence where chemisorbed films initially develop through Fe-S bonding, followed by decomposition (C-O, C-S bonds) and polymerization reactions (S-S, S-O bonds). Thermal and mechanical forces collaboratively facilitate this process. The kinetic analysis demonstrates elevated activation volume exhibited a negative correlation with the reaction frequency difference. Normal stress boosts reactivity by encouraging the polymerization and reducing activation energy. This work offers a comprehensive understanding of alpha-lipoic acid ester tribofilm formation on ferrous interfaces.
Keywords Plus:GLOBAL ENERGY-CONSUMPTION,REACTIVE FORCE-FIELD,MOLECULAR-DYNAMICS,MECHANOCHEMISTRY,PERFORMANCE,FRICTION,REAXFF
Published in TRIBOLOGY INTERNATIONAL,Volume192,10.1016/j.triboint.2024.109291;APR 2024


