Abstract
Solvent effects add a new dimension for tuning the activity and selectivity of heterogeneous catalytic reactions, which is extensively employed in the hydrogenation of unsaturated compounds with multiple functional groups. In this concept, we briefly summarize recent developments on how the solvent effects affect the catalytic performance from the following aspects: 1) the polarity of the solvent can influence the interaction between the solvent and the reactant or intermediate; 2) the composition of mixed solvent can influence the reactivity of the reactant or intermediate; 3) solvent effect varies with the metal identity and support; 4) the solvent can induce surface modification of the supported catalysts. This summarization will provide insights into the rational development of efficient and selective heterogeneous hydrogenation catalytic system to realize the precise synthesis of desired chemicals.
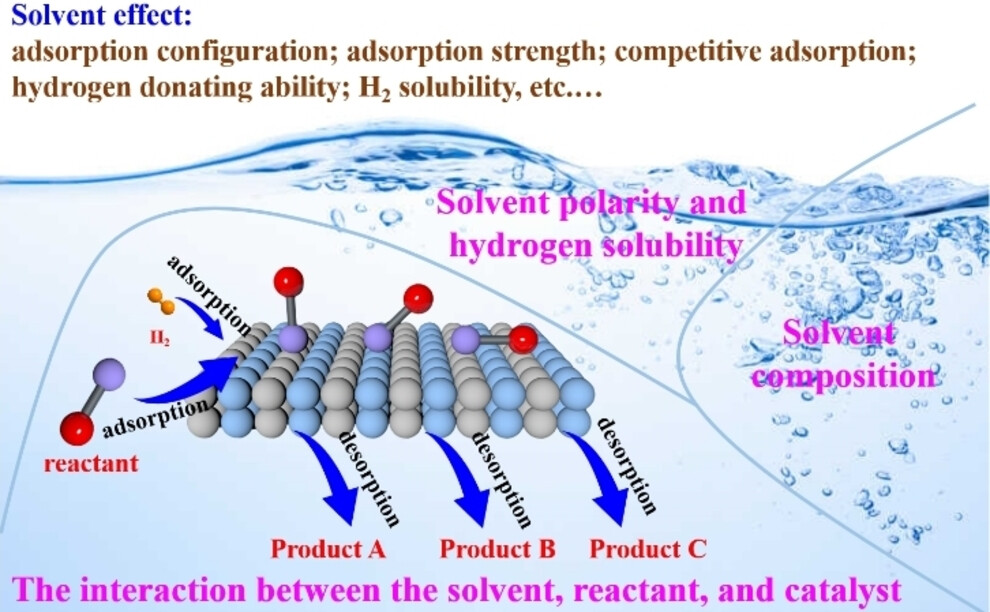
Solvent effects provide numerous opportunities for tuning the activity and selectivity of heterogeneous catalytic reactions. This concept briefly summarizes recent developments on how the solvent effects affect the catalytic performance from solvent polarity, solvent composition, and the interaction between the solvent, reactant and catalysts. image
Keywords Plus:LIQUID-PHASE HYDROGENATION,TRANSITION-METAL,4-PHENYL-2-BUTANONE,CINNAMALDEHYDE,NANOPARTICLES,REACTIVITY,REDUCTION,SOLVATION,POLARITY,SUPPORT
Published in CHEMCATCHEMarrow_drop_down,Volume16;10.1002/cctc.202400120,JUL 22 2024


