Abstract
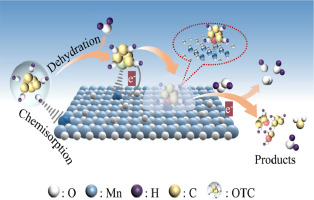
In Fenton -like oxidation, the catalyst directly influences the reaction mechanism for the degradation of pollutants from water. Here, a alpha -MnO 2 catalyst (OAm-1) was synthesized via a self -assembly method with the assistance of a surfactant. OAm-1 possessed a large specific surface area of 221 m 2 /g, abundant mesoporous structures and a large proportion of Mn(III). Further characterization exhibited that OAm-1 had abundant oxygen vacancies and excellent reducibility and conductivity. The adsorption and catalytic ability of OAm-1 were studied in the degradation of oxytetracycline (OTC) via the activation of hydrogen peroxide (H 2 O 2 ). Through the radical quenching experiments, electron resonance spectroscopy (EPR), Xray photoelectron spectroscopy (XPS) and Fourier -transform infrared spectroscopy (FT -IR) analysis, Mn(III) of OAm-1 was proved to be the active sites for the chemisorption of OTC. Systematic electrochemical experiments and analysis have shown that a process of electron transfer mediated by OAm-1 occurred between the pollutant and H 2 O 2 during a Fenton -like reaction. This work experimentally verifies the electron transfer process dominated nonradical mechanism over alpha -MnO 2 , which is helpful for understanding the catalytic mechanism of the Fenton -like oxidation. (c) 2024 Published by Elsevier B.V. on behalf of Chinese Chemical Society and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Keywords Plus:ORGANIC CONTAMINANTS,ACTIVATION,OXIDATION,PEROXYMONOSULFATE,ANTIBIOTICS,EFFICIENCY,REMOVAL,BIOCHAR,H2O2
Published in CHINESE CHEMICAL LETTERS,Volume 35;10.1016/j.cclet.2023.109331,AUG 2024


